THE AZ STUDY: Why did AZ asked to withdraw their EUA marketing authorisation approval, and how come you didn't hear about it?
Short answer: BECAUSE THEIR LONG TERM SAFETY STUDY SHOWN THAT THEIR COV!D19 SH0TS K!LLED AND HARMED PEOPLE.
Long answer: READ THE THREAD!
#TheAZStudy
1/
Short answer: BECAUSE THEIR LONG TERM SAFETY STUDY SHOWN THAT THEIR COV!D19 SH0TS K!LLED AND HARMED PEOPLE.
Long answer: READ THE THREAD!
#TheAZStudy
1/
2/
As you might have heard, AstraZeneca ASKED in March 2024 to have their COV!D19 SH0TS product approval withdrawn from Europe's EMA. It was not the European Union (EMA) who decided to withdraw their approval due to the damage they caused people. BUT WHY?
ec.europa.eu
As you might have heard, AstraZeneca ASKED in March 2024 to have their COV!D19 SH0TS product approval withdrawn from Europe's EMA. It was not the European Union (EMA) who decided to withdraw their approval due to the damage they caused people. BUT WHY?
ec.europa.eu
3/
To understand what happened, let's start with a QUICK, SHORT reminder (the following 3 posts) before we will go into the details.
I promise you it's worth reading!
29 JANUARY 2021: EMA recommends COVID-19 Vaccine AstraZeneca for authorisation in the EU
ema.europa.eu
To understand what happened, let's start with a QUICK, SHORT reminder (the following 3 posts) before we will go into the details.
I promise you it's worth reading!
29 JANUARY 2021: EMA recommends COVID-19 Vaccine AstraZeneca for authorisation in the EU
ema.europa.eu
4/
11 March 2021:
EME's Pharmacovigilance Risk Assessment Committee (PRAC) investigated cases of thromboembolic events, but claimed the vaccine’s benefits currently still outweigh risks.
Denmark, Norway, and Iceland suspended use of the product.
ema.europa.eu
11 March 2021:
EME's Pharmacovigilance Risk Assessment Committee (PRAC) investigated cases of thromboembolic events, but claimed the vaccine’s benefits currently still outweigh risks.
Denmark, Norway, and Iceland suspended use of the product.
ema.europa.eu
5/
31 MARCH 2021:
Many EU countries resumed the use of AZ products, but restricted it to people above certain age.
aljazeera.com
31 MARCH 2021:
Many EU countries resumed the use of AZ products, but restricted it to people above certain age.
aljazeera.com
6/
BACK TO THE STUDY. The EMA has requested AZ to perform multiple risk assessments of their products. It is all mentioned here.
ema.europa.eu
BACK TO THE STUDY. The EMA has requested AZ to perform multiple risk assessments of their products. It is all mentioned here.
ema.europa.eu
8/
The study started on 28 Aug 2020, it's primary completion date was supposed to be 05 Mar 2021, reported to the EU Clinical Trials Register as completed on 21 Mar 2023 and submitted 23 Nov 2023.
clinicaltrialsregister.eu
The study started on 28 Aug 2020, it's primary completion date was supposed to be 05 Mar 2021, reported to the EU Clinical Trials Register as completed on 21 Mar 2023 and submitted 23 Nov 2023.
clinicaltrialsregister.eu
9/
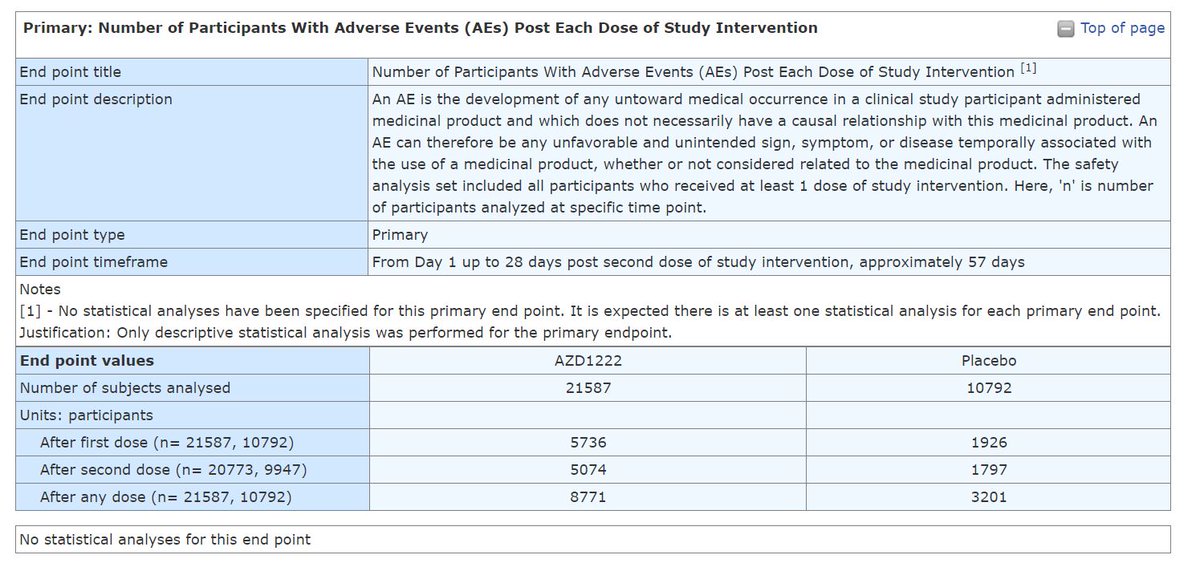
Notice that the placebo group is about 50% in size of the group that received the product AZ (AZD1222).
Notice that the placebo group is about 50% in size of the group that received the product AZ (AZD1222).
11/
Notice the statement: "No statistical analyses have been specified for this primary end point. It is expected there is at least one statistical analysis for each primary end point.
Justification: Only descriptive statistical analysis was performed for the primary endpoint."
Notice the statement: "No statistical analyses have been specified for this primary end point. It is expected there is at least one statistical analysis for each primary end point.
Justification: Only descriptive statistical analysis was performed for the primary endpoint."
13/
YOU DO NOT NEED TO BE A STATISTICIAN TO SEE THAT THE EVENTS IN THOSE WHO RECEIVED THE AZ COV!D19 SH0TS GROUP ARE WAY MORE THAN DOUBLE THAN IN THE PLACEBO.
I'll let statisticians do the math...
YOU DO NOT NEED TO BE A STATISTICIAN TO SEE THAT THE EVENTS IN THOSE WHO RECEIVED THE AZ COV!D19 SH0TS GROUP ARE WAY MORE THAN DOUBLE THAN IN THE PLACEBO.
I'll let statisticians do the math...
15/
This study was reported on the Committee for medicinal products for human use (CHMP) meeting that took place between 18-21 March 2024, with "Positive Opinion adopted by consensus on 07.03.2024."
ema.europa.eu
This study was reported on the Committee for medicinal products for human use (CHMP) meeting that took place between 18-21 March 2024, with "Positive Opinion adopted by consensus on 07.03.2024."
ema.europa.eu
17/
The timeline of this research and its results indicates why AstraZeneca has decided to apply for the withdrawal of their marketing authorisation.
Next time someone tells you that something is "Safe and effective", remind them this study that showed no long term safety.
The timeline of this research and its results indicates why AstraZeneca has decided to apply for the withdrawal of their marketing authorisation.
Next time someone tells you that something is "Safe and effective", remind them this study that showed no long term safety.
18/
Adverse Event of Special Interest (AESI) group is defined by the researchers, which means that events such as myocarditis which normally be described as SAEs could be defined as AESIs, which allows the researchers to claimed they have a low amount of Serious Adverse Events.
Adverse Event of Special Interest (AESI) group is defined by the researchers, which means that events such as myocarditis which normally be described as SAEs could be defined as AESIs, which allows the researchers to claimed they have a low amount of Serious Adverse Events.
19/
Do we know what is the EMA POSITION on the safety of this product NOW THAT THEY GOT THE LONG TERM STUDY RESULTS? Oh wait, they don’t need to tell us. The product has been withdrawn so no need to look further. How continent for AstraZeneca and the EMA.
Simply unbelievable!
Do we know what is the EMA POSITION on the safety of this product NOW THAT THEY GOT THE LONG TERM STUDY RESULTS? Oh wait, they don’t need to tell us. The product has been withdrawn so no need to look further. How continent for AstraZeneca and the EMA.
Simply unbelievable!
Loading suggestions...