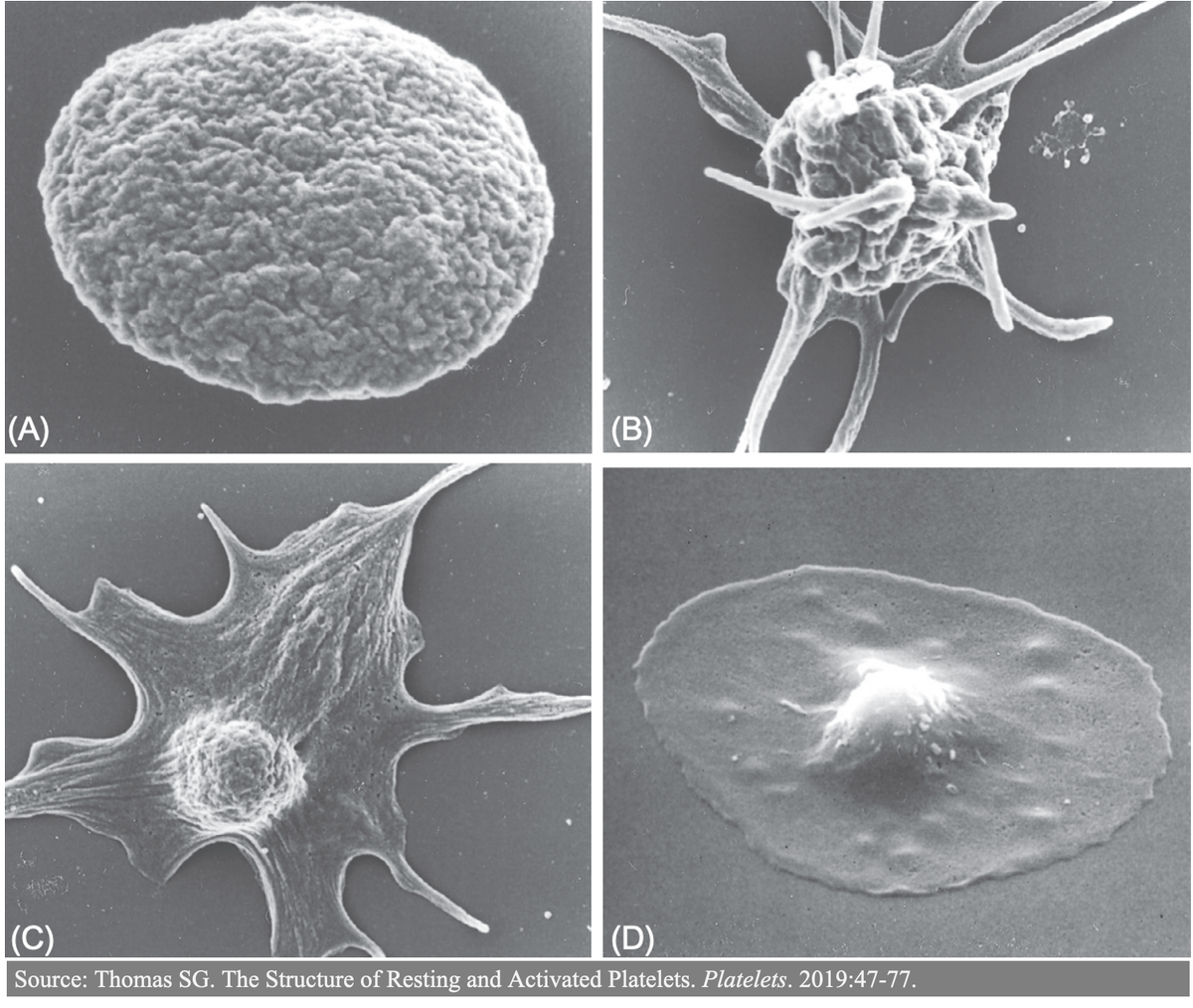
2/
Just about every aspect of primary hemostasis is affected in uremia. For this thread, I'll focus on how toxins disrupt:
▪️Adhesion (attachment of platelets to exposed subendothelial collagen)
▪️Aggregation (attachment of platelets to each other)
pubmed.ncbi.nlm.nih.gov
Just about every aspect of primary hemostasis is affected in uremia. For this thread, I'll focus on how toxins disrupt:
▪️Adhesion (attachment of platelets to exposed subendothelial collagen)
▪️Aggregation (attachment of platelets to each other)
pubmed.ncbi.nlm.nih.gov
3/
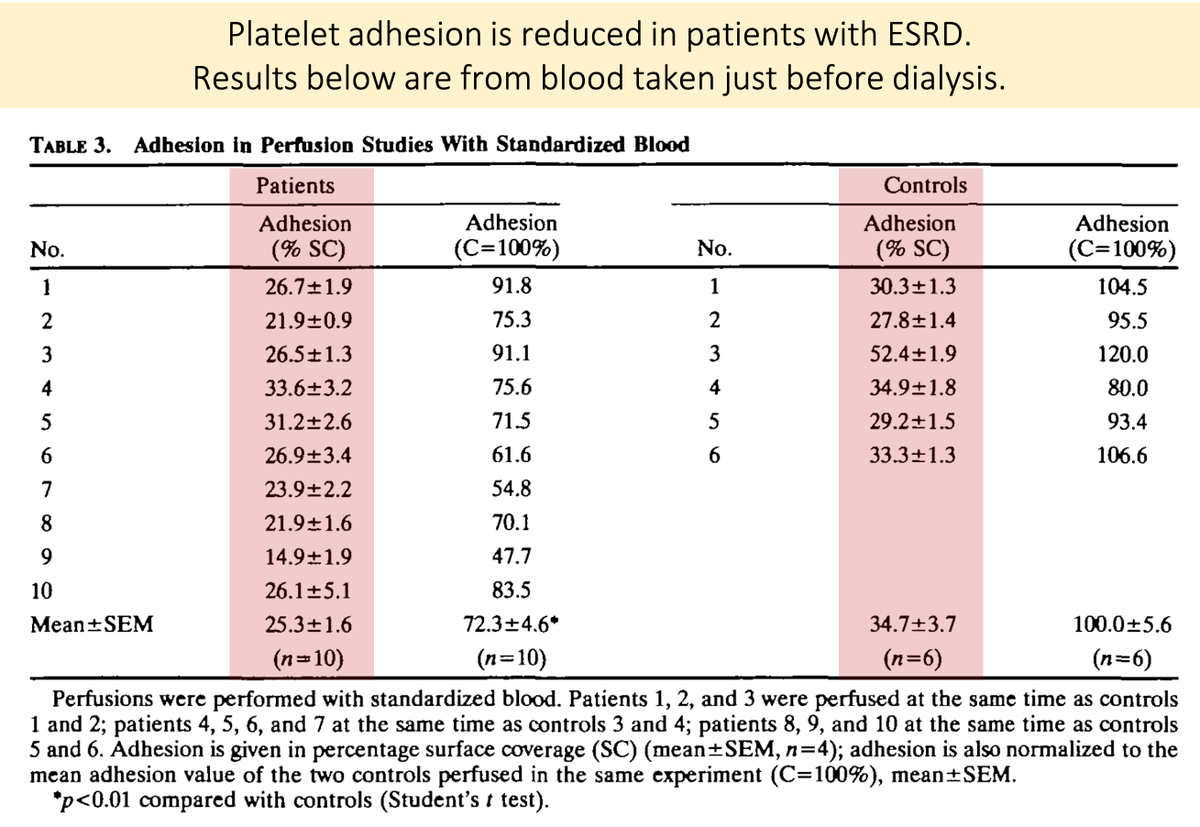
One study took blood from patients with ESRD just before dialysis and found reductions in platelet adhesion and aggregation.
When normal platelets were placed in uremic plasma, the results were similar.
🔑Something in the plasma causes the problem!
pubmed.ncbi.nlm.nih.gov
One study took blood from patients with ESRD just before dialysis and found reductions in platelet adhesion and aggregation.
When normal platelets were placed in uremic plasma, the results were similar.
🔑Something in the plasma causes the problem!
pubmed.ncbi.nlm.nih.gov
4/
There are many potential "uremic toxins".
Which do you suspect is the culprit causing bleeding problems in patients with renal failure?
There are many potential "uremic toxins".
Which do you suspect is the culprit causing bleeding problems in patients with renal failure?
5/
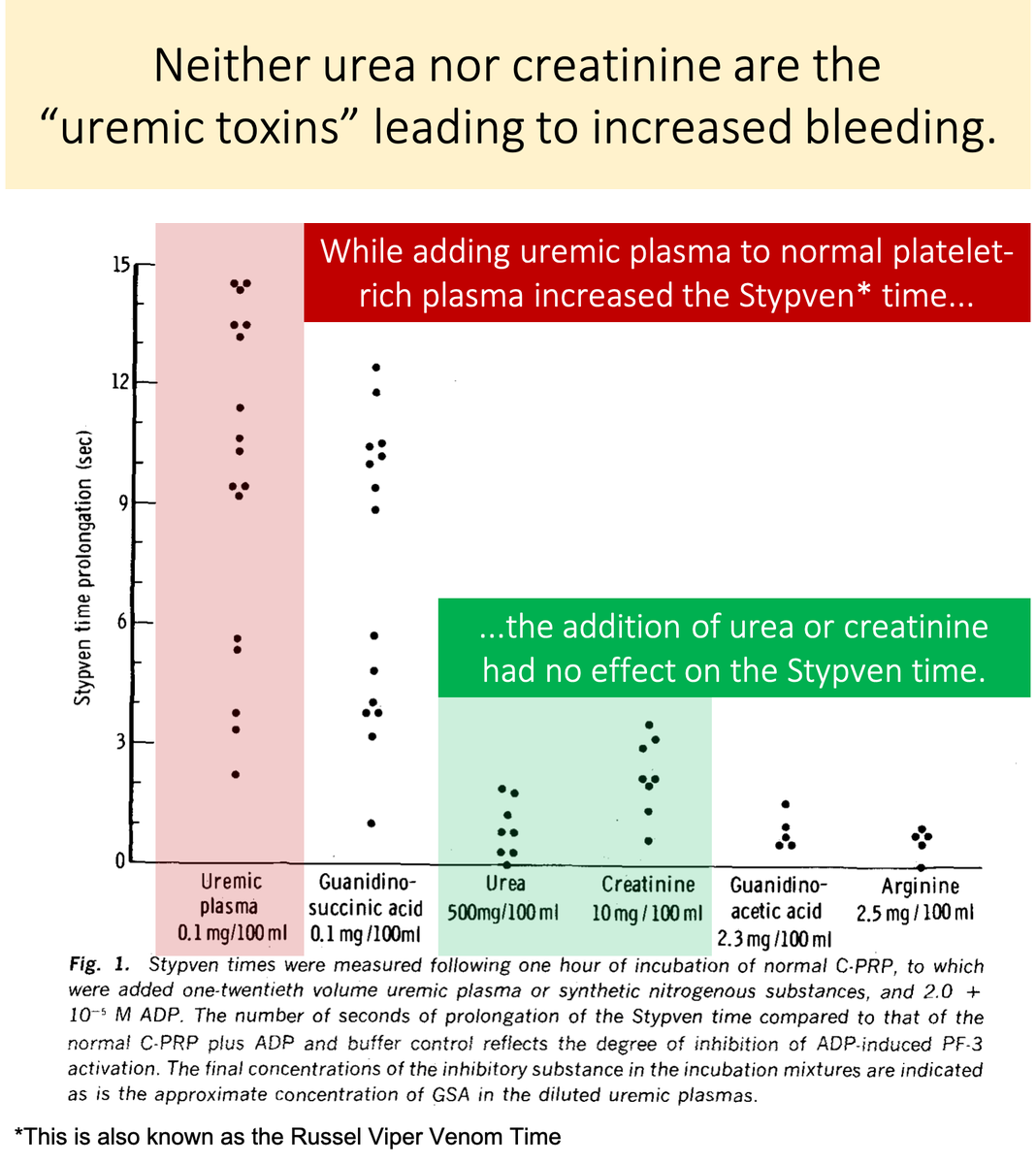
Neither urea nor creatinine cause uremic platelets.
In one study their addition to normal platelet-rich plasma had no effect on platelet function.
The toxin is something other than urea or creatinine!
pubmed.ncbi.nlm.nih.gov
Neither urea nor creatinine cause uremic platelets.
In one study their addition to normal platelet-rich plasma had no effect on platelet function.
The toxin is something other than urea or creatinine!
pubmed.ncbi.nlm.nih.gov
6/
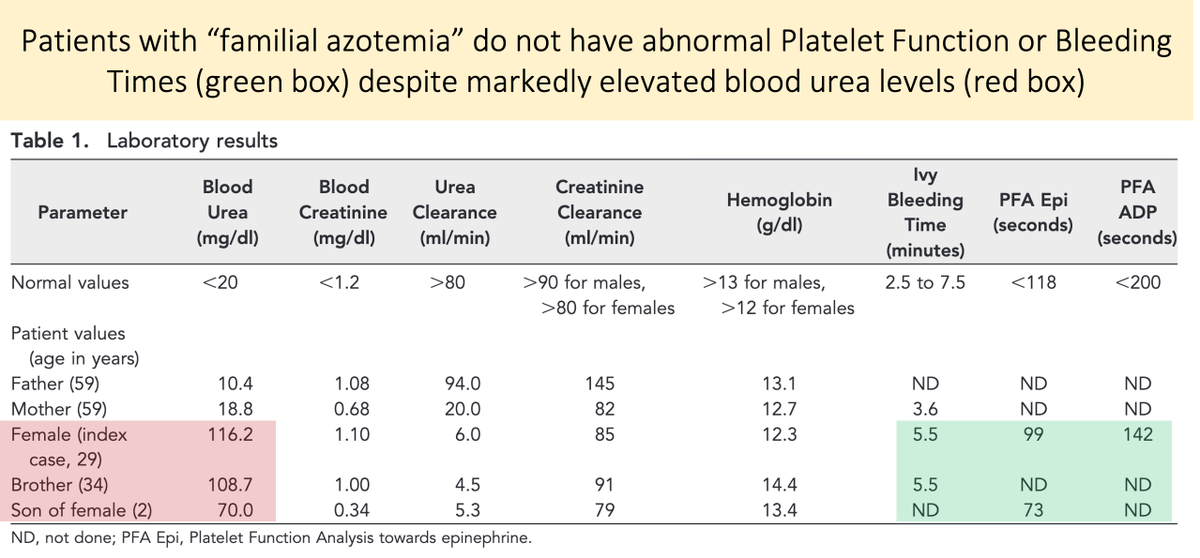
The lack of a role of urea as a uremic toxin is supported by the study of familial azotemia (a syndrome characterized by high plasma urea but normal renal function).
These patients do not experience uremic symptoms and have normal platelet function.
ncbi.nlm.nih.gov
The lack of a role of urea as a uremic toxin is supported by the study of familial azotemia (a syndrome characterized by high plasma urea but normal renal function).
These patients do not experience uremic symptoms and have normal platelet function.
ncbi.nlm.nih.gov
7/
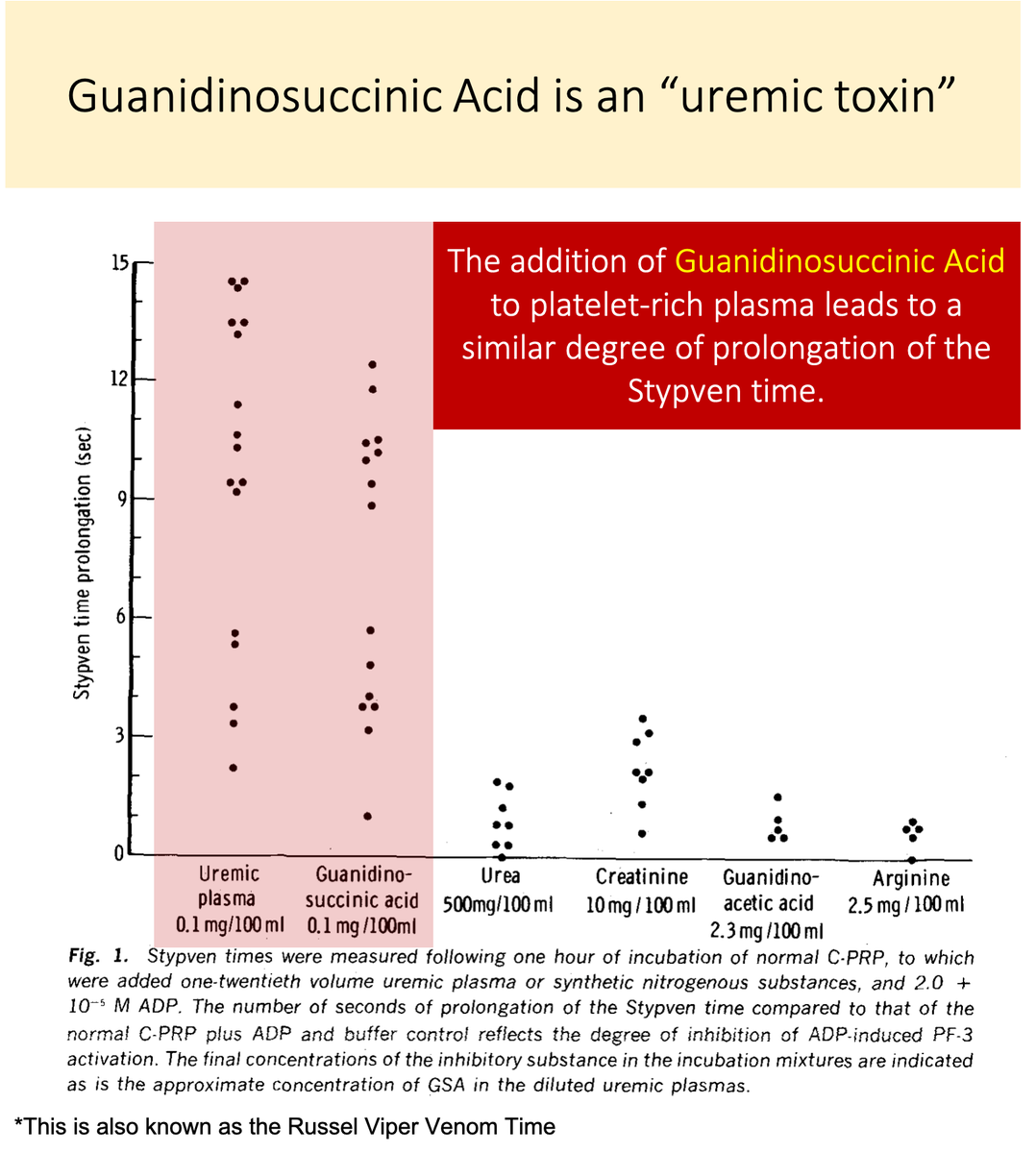
Let's look again at the study in tweet 5.
You'll notice that there IS a substance that, when added to normal platelet-rich plasma, negatively affects platelet function.
💥Guanidinosuccinic acid💥
pubmed.ncbi.nlm.nih.gov
Let's look again at the study in tweet 5.
You'll notice that there IS a substance that, when added to normal platelet-rich plasma, negatively affects platelet function.
💥Guanidinosuccinic acid💥
pubmed.ncbi.nlm.nih.gov
8/
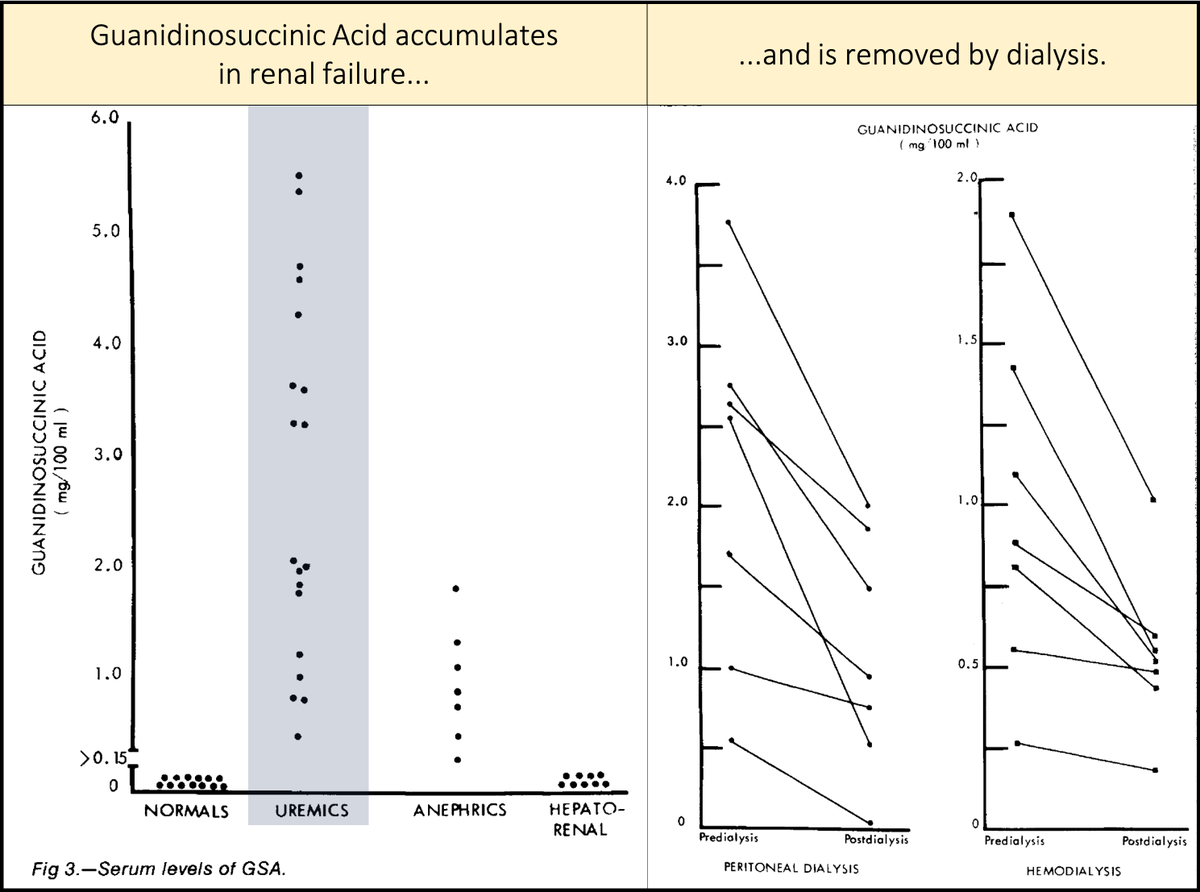
Key points about guanidinosuccinic acid (GSA):
▪️It accumulates in renal failure
▪️Is removed by dialysis
[Note: though not all studies agree, dialysis is generally felt to mitigate platelet dysfunction by removal of a toxin.]
pubmed.ncbi.nlm.nih.gov
Key points about guanidinosuccinic acid (GSA):
▪️It accumulates in renal failure
▪️Is removed by dialysis
[Note: though not all studies agree, dialysis is generally felt to mitigate platelet dysfunction by removal of a toxin.]
pubmed.ncbi.nlm.nih.gov
9/
We now have two questions:
1⃣Why does GSA accumulate?
&
2⃣What does it do to platelets?
We now have two questions:
1⃣Why does GSA accumulate?
&
2⃣What does it do to platelets?
10/
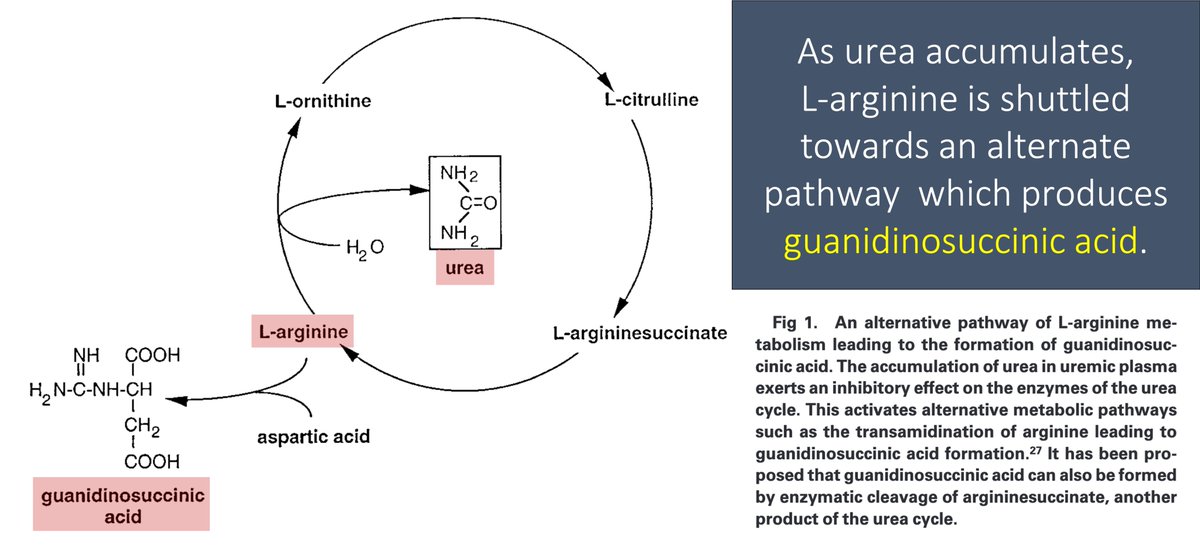
In renal failure, urea accumulates in the blood. As a result, the enzymes that convert ammonia to urea (the urea cycle!) are down-regulated.
As an alternative pathway to deal with toxic ammonia, L-arginine is converted to GSA.
GSA levels rise.
pubmed.ncbi.nlm.nih.gov
In renal failure, urea accumulates in the blood. As a result, the enzymes that convert ammonia to urea (the urea cycle!) are down-regulated.
As an alternative pathway to deal with toxic ammonia, L-arginine is converted to GSA.
GSA levels rise.
pubmed.ncbi.nlm.nih.gov
11/
To understand why GSA leads to platelet dysfunction, we must briefly review how nitric oxide (NO) affects hemostasis.
In addition to causing vasodilation (which helps promote blood flow and reduce hemostasis), NO also inhibits platelet function.
pubmed.ncbi.nlm.nih.gov
To understand why GSA leads to platelet dysfunction, we must briefly review how nitric oxide (NO) affects hemostasis.
In addition to causing vasodilation (which helps promote blood flow and reduce hemostasis), NO also inhibits platelet function.
pubmed.ncbi.nlm.nih.gov
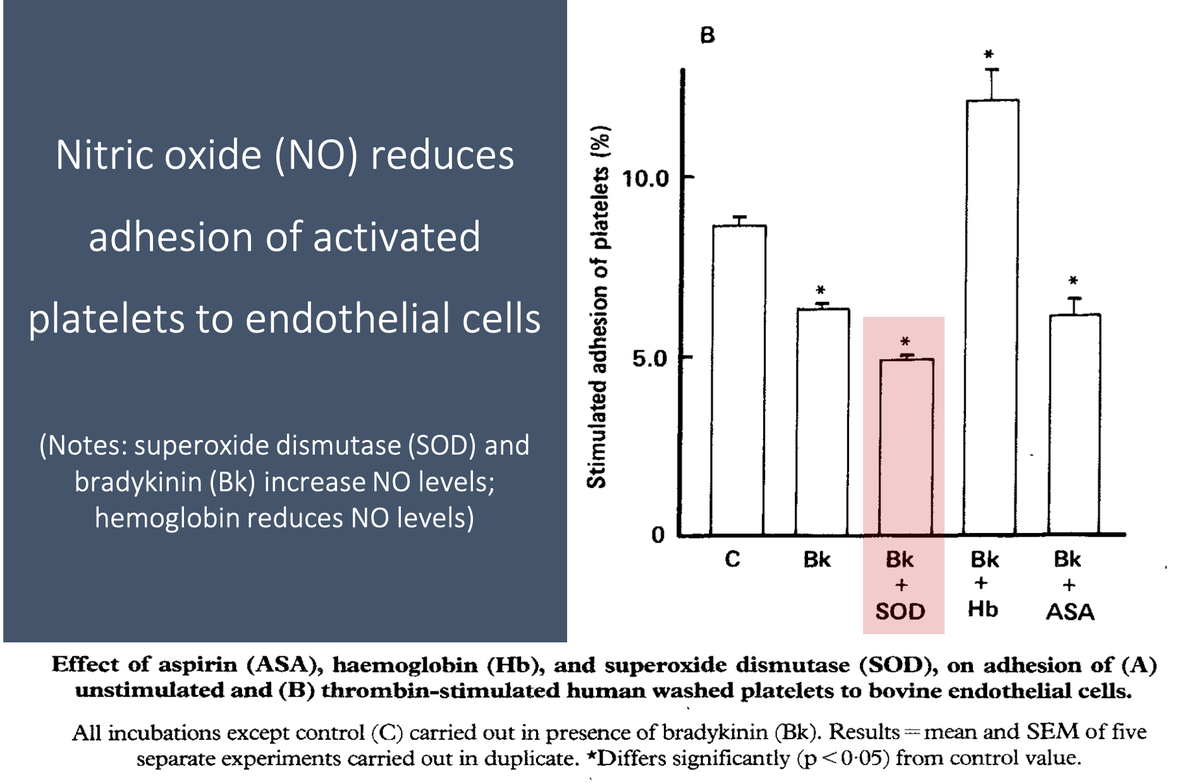
12/
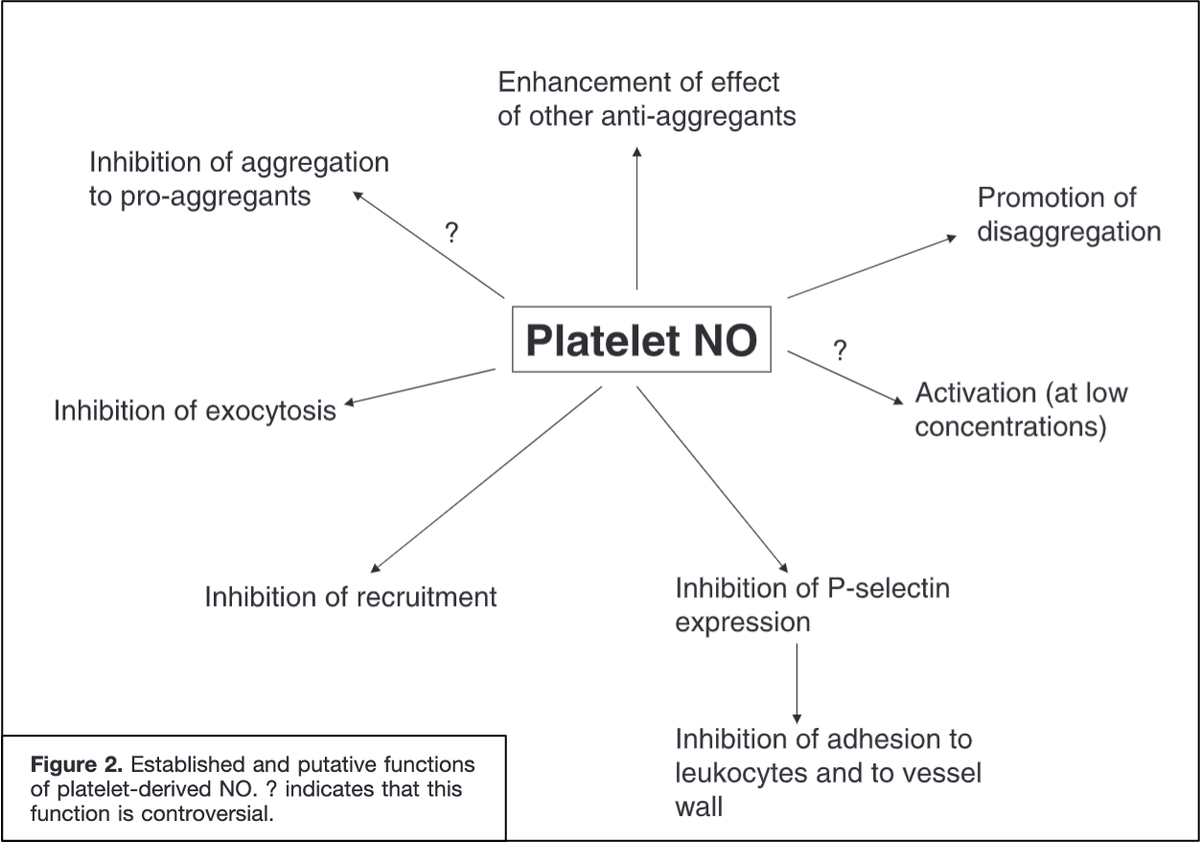
For example, nitric oxide reduces platelet adhesion to the endothelium (Link 1 and Picture) and platelet-platelet aggregation.
These effects are mediated by an increase in cyclic GMP (Link 2).
pubmed.ncbi.nlm.nih.gov
pubmed.ncbi.nlm.nih.gov
For example, nitric oxide reduces platelet adhesion to the endothelium (Link 1 and Picture) and platelet-platelet aggregation.
These effects are mediated by an increase in cyclic GMP (Link 2).
pubmed.ncbi.nlm.nih.gov
pubmed.ncbi.nlm.nih.gov
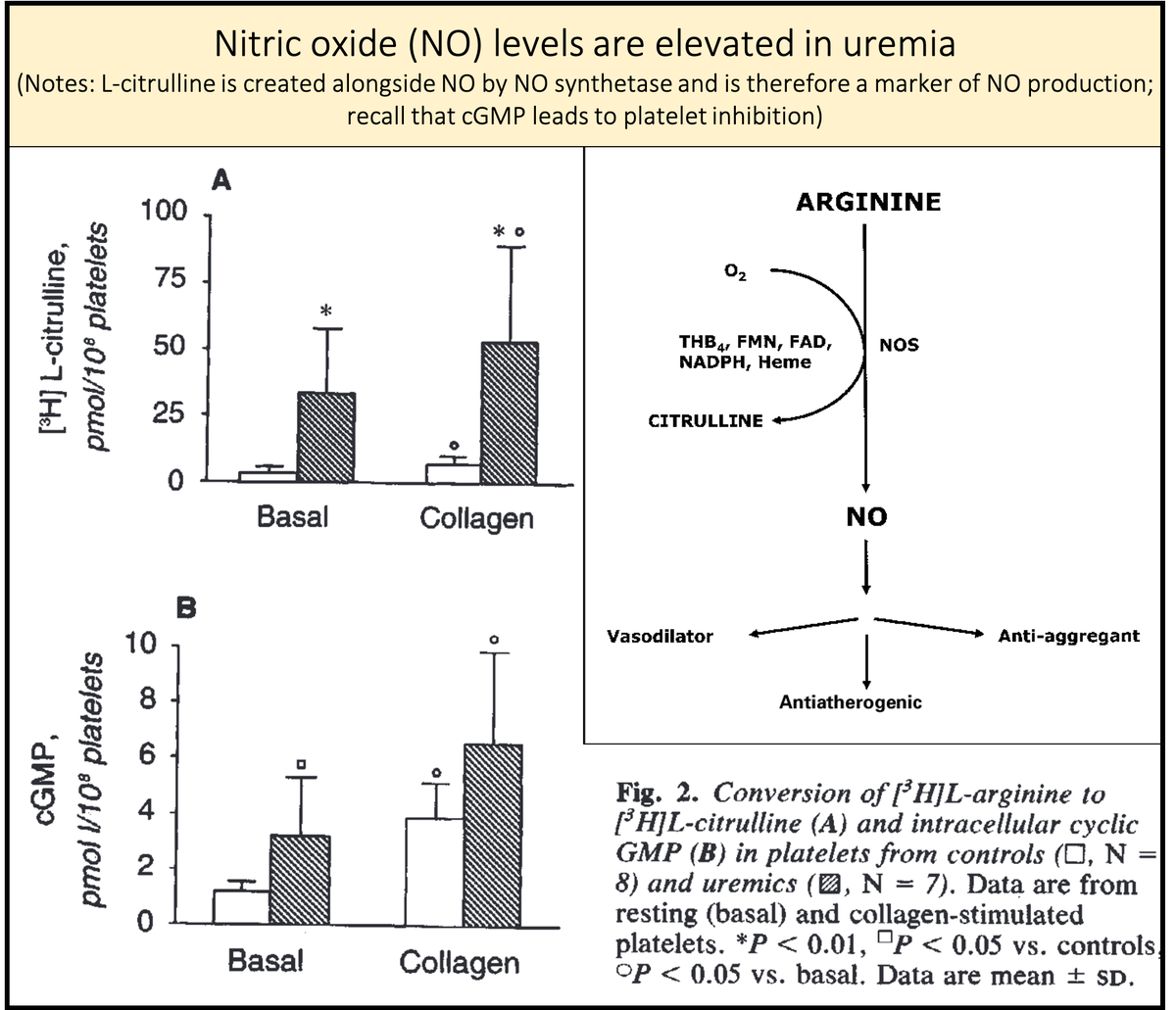
13/
And how do these effects of nitric oxide relate to uremia and increased levels of GSA?
First, nitric oxide synthesis is enhanced in uremia. This may be the end-product leading to many of the features of uremic platelets.
pubmed.ncbi.nlm.nih.gov
And how do these effects of nitric oxide relate to uremia and increased levels of GSA?
First, nitric oxide synthesis is enhanced in uremia. This may be the end-product leading to many of the features of uremic platelets.
pubmed.ncbi.nlm.nih.gov
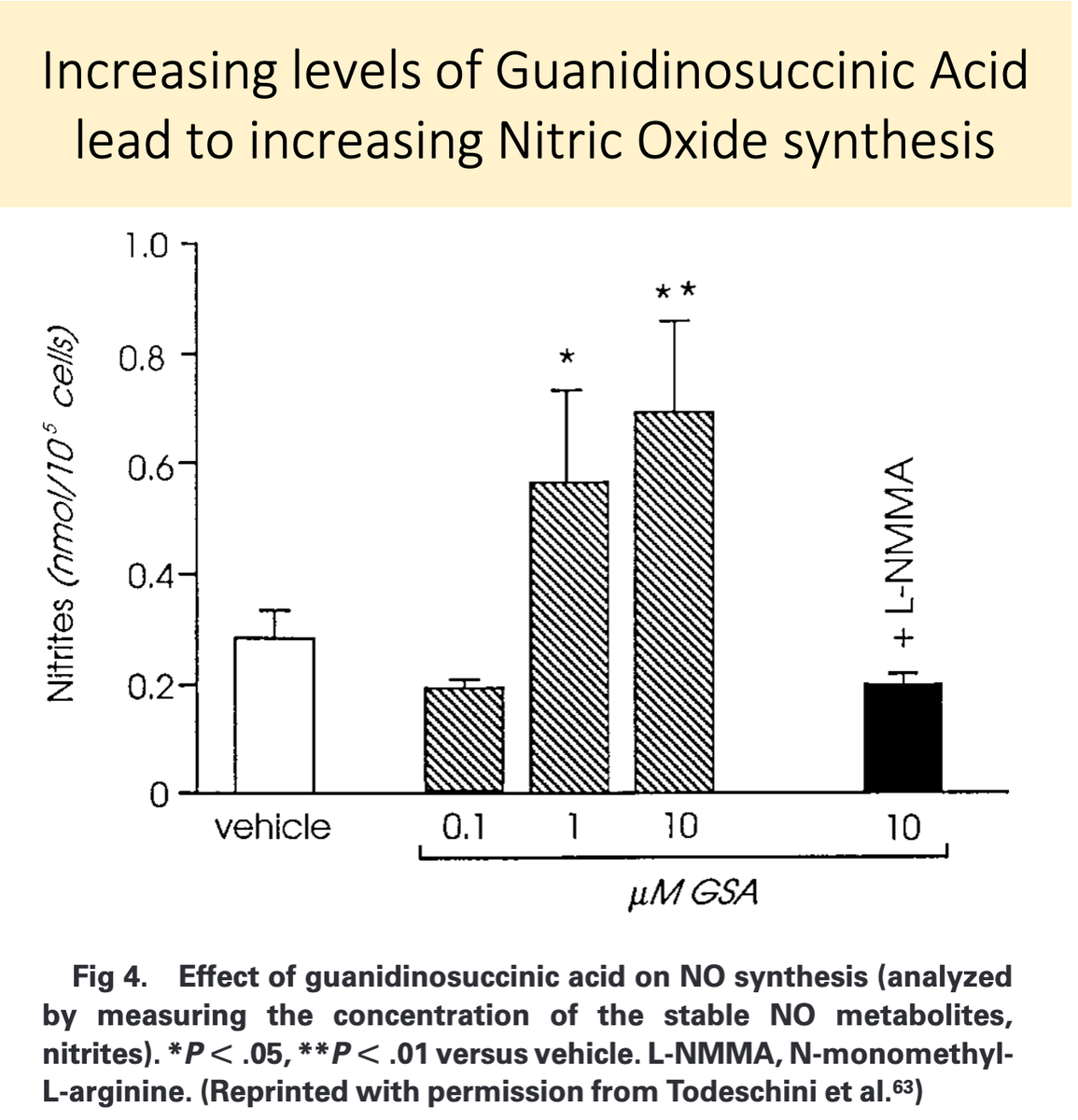
14/
Second, because GSA is structurally similar to L-arginine, it is likely converted to nitric oxide in uremia.
GSA accumulates in renal failure and is converted to nitric oxide, leading to "uremic platelets"!
pubmed.ncbi.nlm.nih.gov
Second, because GSA is structurally similar to L-arginine, it is likely converted to nitric oxide in uremia.
GSA accumulates in renal failure and is converted to nitric oxide, leading to "uremic platelets"!
pubmed.ncbi.nlm.nih.gov
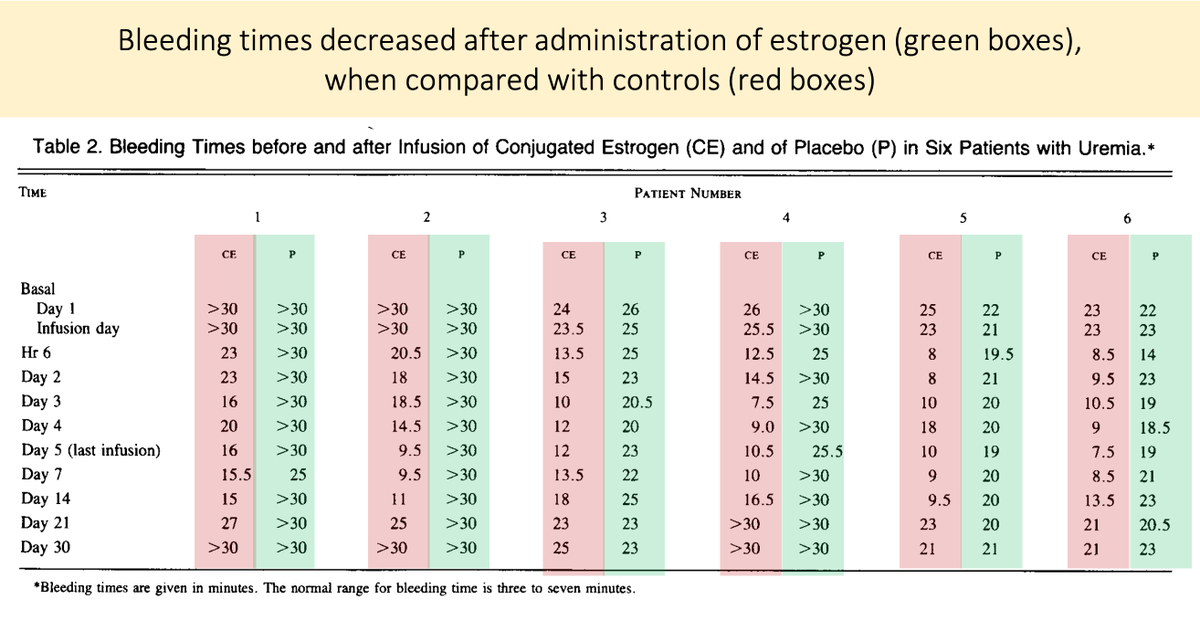
15/
Before closing, there is an interesting clinical correlate.
Estrogens are an effective treatment for uremic bleeding (Link 1 and Picture).
The proposed mechanism? Estrogen-mediated reduction in nitric oxide! (Link 2).
pubmed.ncbi.nlm.nih.gov
pubmed.ncbi.nlm.nih.gov
Before closing, there is an interesting clinical correlate.
Estrogens are an effective treatment for uremic bleeding (Link 1 and Picture).
The proposed mechanism? Estrogen-mediated reduction in nitric oxide! (Link 2).
pubmed.ncbi.nlm.nih.gov
pubmed.ncbi.nlm.nih.gov
16/16
◼️Guanidinosuccinic acid (GSA) accumulates in renal failure
◼️Increased GSA leads to increased nitric oxide (NO)
◼️NO has multiple antiplatelet effects
💥GSA is a uremic toxic that leads to uremic platelets
[Reminder: there are MANY other mechanisms; see tweet 2.]
◼️Guanidinosuccinic acid (GSA) accumulates in renal failure
◼️Increased GSA leads to increased nitric oxide (NO)
◼️NO has multiple antiplatelet effects
💥GSA is a uremic toxic that leads to uremic platelets
[Reminder: there are MANY other mechanisms; see tweet 2.]
I want to extend a HUGE thank you to...
🎗️Justine Ryu (@justine_ryu), Heme/Onc Fellow, Division of Hematology & Oncology, @BIDMChealth
🎗️Jason Freed (@FreedoBaggins), Hematologist, Division of Hematology & Oncology, @BIDMChealth
...for their peer review of this tweetorial!
🎗️Justine Ryu (@justine_ryu), Heme/Onc Fellow, Division of Hematology & Oncology, @BIDMChealth
🎗️Jason Freed (@FreedoBaggins), Hematologist, Division of Hematology & Oncology, @BIDMChealth
...for their peer review of this tweetorial!
Very appropriate question. From what I understand, there has been a lot of discussion about the appropriateness of VKA and DOACs in ESRD.
As with anything, it is a risk/benefit issue.
Here is a recent study on warfarin.
jamanetwork.com
As with anything, it is a risk/benefit issue.
Here is a recent study on warfarin.
jamanetwork.com
This is a great thought, Avi. I simplistically viewed DDAVP as increasing VFW, but this connection with nitric oxide is intriguing!
Another great clinical correlate. Thank you adding, Jason.
Absolutely fascinating!
Loading suggestions...