#ESC20 PARALLAX Trial 🧵
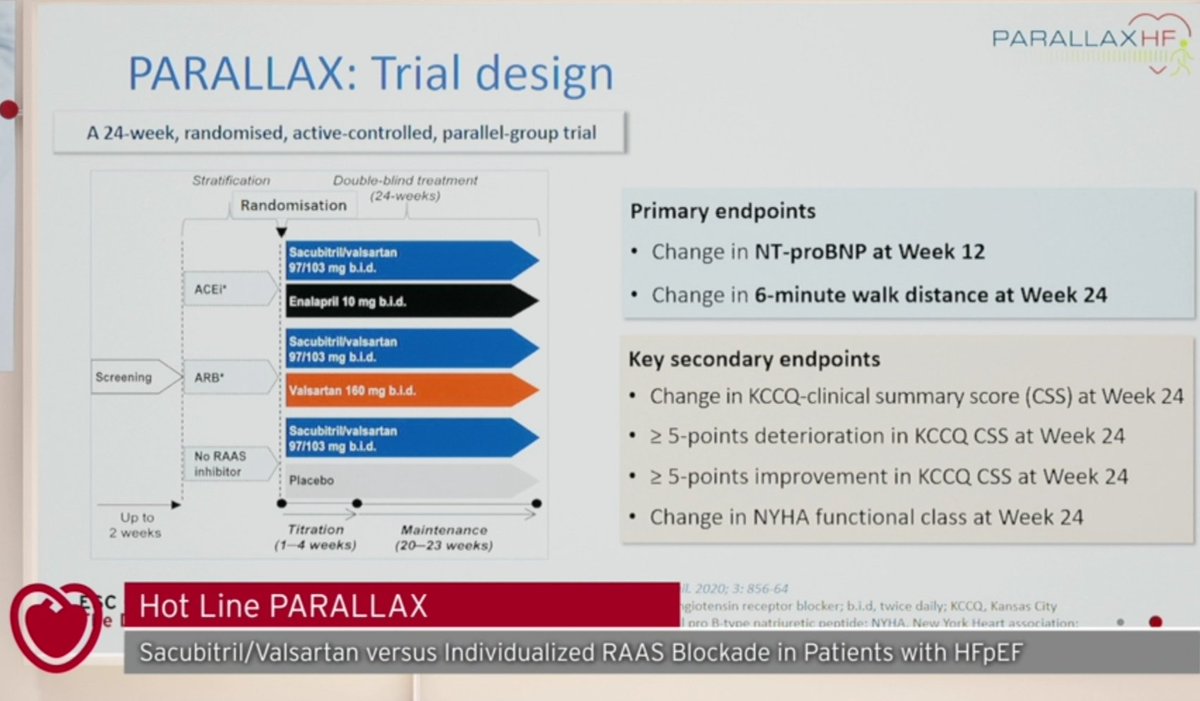
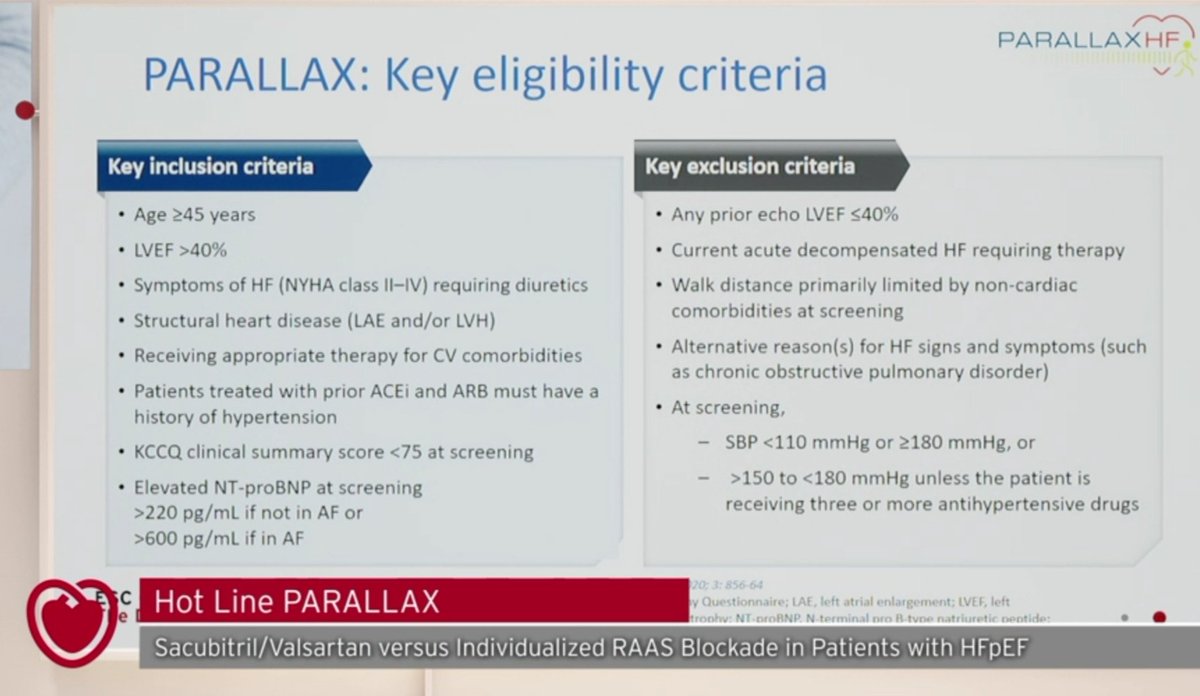
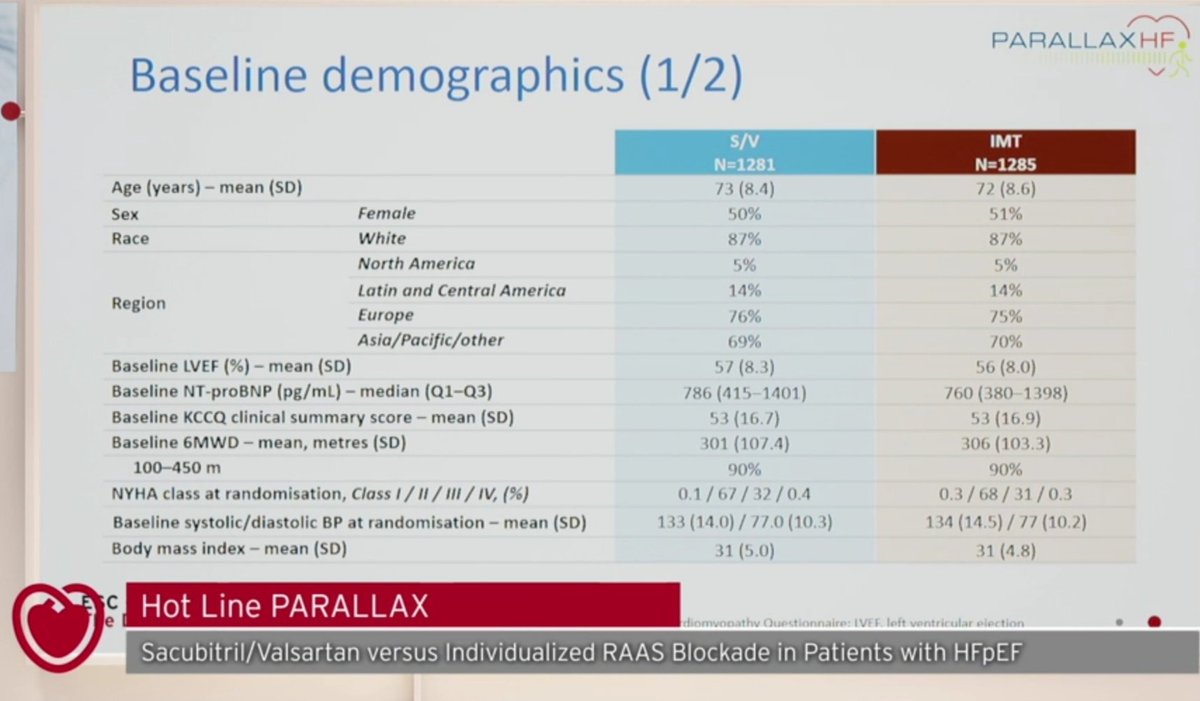
-2,566 HF patients (LVEF >40%)
-Sacubitril-Valsartan vs Active-controlled treatment (Enalapril, Valsartan or Placebo)
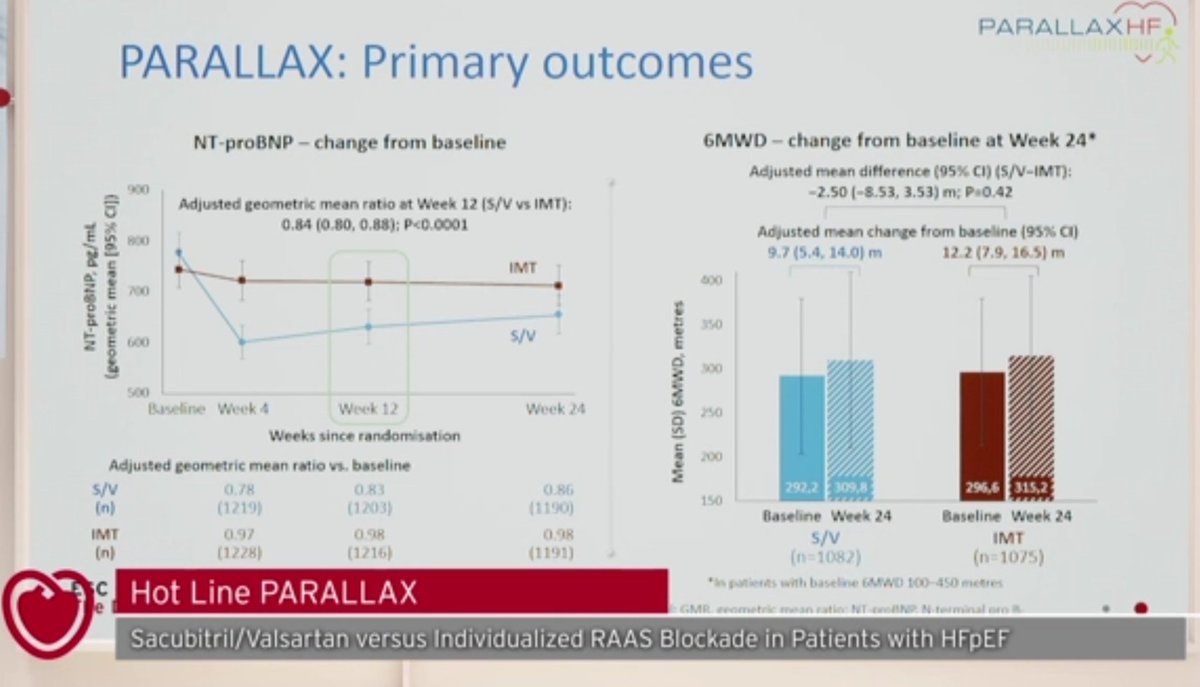
-Primary endpoint: NTproBNP at 12 weeks and 6MWD at 24 weeks
-Exploratory endpoints: eGFR change, HF hospitalization and HF death
-2,566 HF patients (LVEF >40%)
-Sacubitril-Valsartan vs Active-controlled treatment (Enalapril, Valsartan or Placebo)
-Primary endpoint: NTproBNP at 12 weeks and 6MWD at 24 weeks
-Exploratory endpoints: eGFR change, HF hospitalization and HF death
Conclusions
-Trial with mixed results
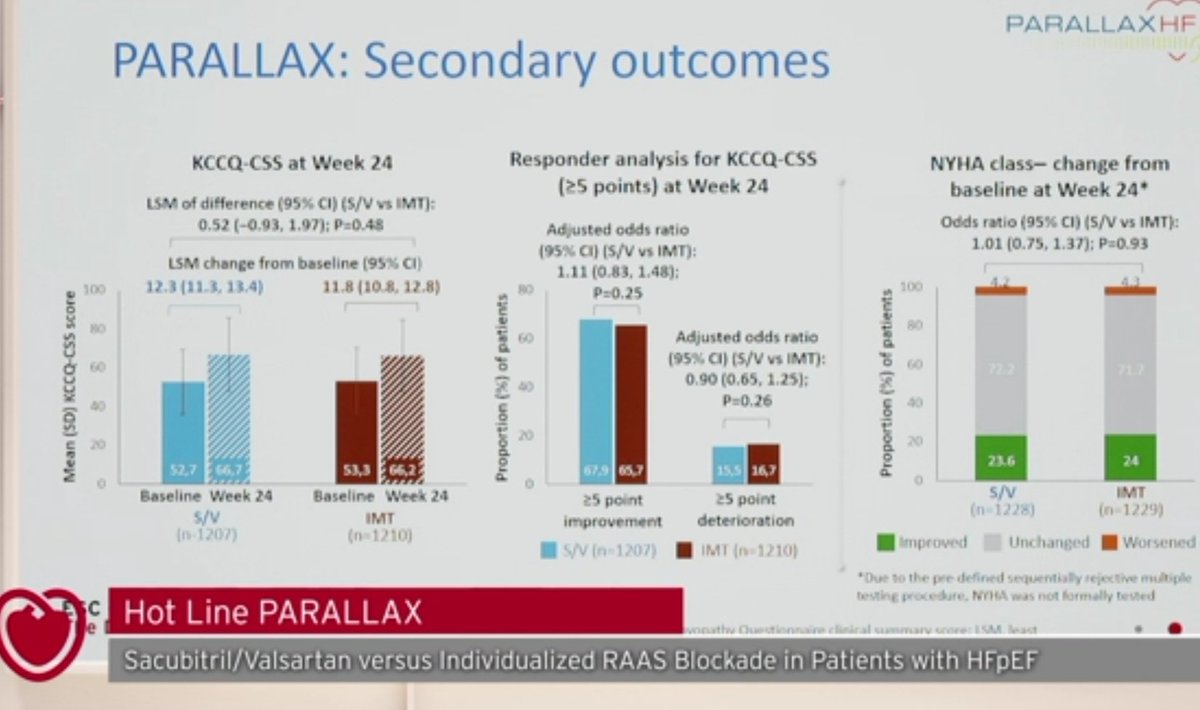
-Without difference in quality of life
-Significant difference in "soft" primary endpoint (NTproBNP at 12 w)
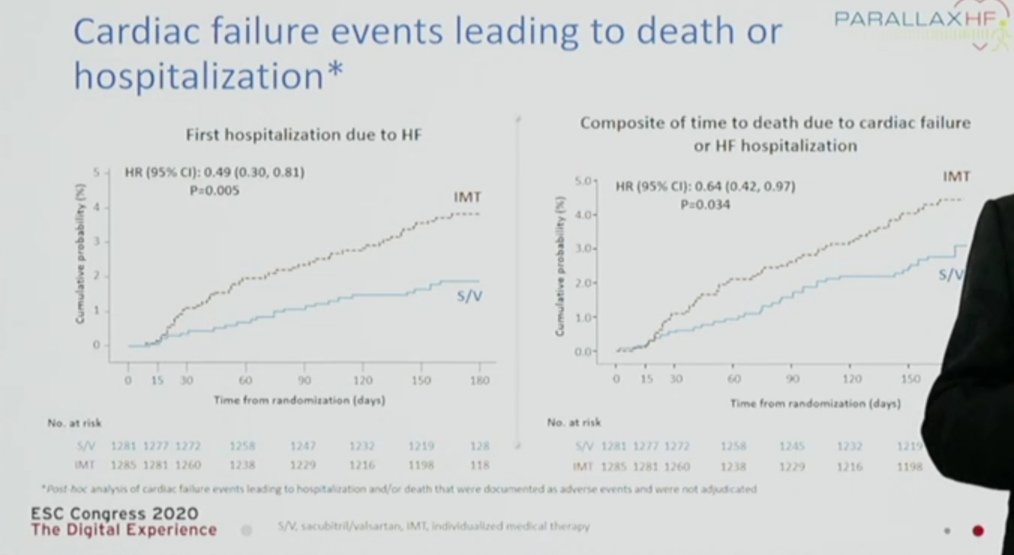
-Significant difference in "hard" exploratory endpoints (HF outcomes)
-Confirms PARAGON-HF trends
-Finally a treatment for HFpEF?
-Trial with mixed results
-Without difference in quality of life
-Significant difference in "soft" primary endpoint (NTproBNP at 12 w)
-Significant difference in "hard" exploratory endpoints (HF outcomes)
-Confirms PARAGON-HF trends
-Finally a treatment for HFpEF?
Loading suggestions...