Education
Health
Healthcare
Investing
Diseases
Pharmaceuticals
manufacturing
Drugs
Pharma Companies
US FDA Terminology
Thread on common terminologies one should know while researching pharma companies
Like and retweet for better reach ! 🧪💊🩺💉
@unseenvalue
Topics Covered:
Diseases
1.1 Acute Disease
1.2 Chronic Disease
1.3 Orphan Disease
Drugs
2.1 Pharmaceutical Drugs
2.2 Biologic Drug
Like and retweet for better reach ! 🧪💊🩺💉
@unseenvalue
Topics Covered:
Diseases
1.1 Acute Disease
1.2 Chronic Disease
1.3 Orphan Disease
Drugs
2.1 Pharmaceutical Drugs
2.2 Biologic Drug
2.3 Biosimilar
2.4 Bioequivalence
Manufacturing
3.1 Intermediate
3.2 API
3.3 Formulation
US FDA Terminology
4.1 DMF
4.2 VMF
4.3 NDA
4.4 ANDA
4.5 Orange book
4.6 Purple book
4.7 Green book
2.4 Bioequivalence
Manufacturing
3.1 Intermediate
3.2 API
3.3 Formulation
US FDA Terminology
4.1 DMF
4.2 VMF
4.3 NDA
4.4 ANDA
4.5 Orange book
4.6 Purple book
4.7 Green book
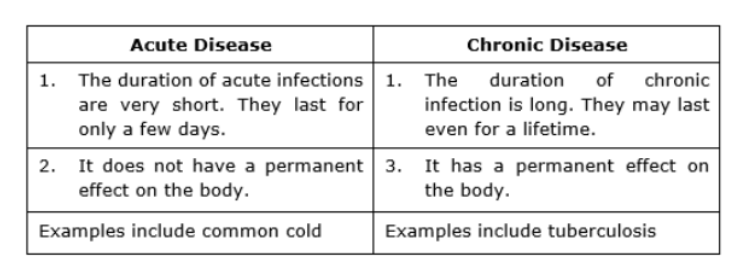
1.2 Chronic Disease: Chronic diseases are long lasting diseases which require constant medical attention and need for medication. Chronic diseases severely impact a patient’s daily activities and in most cases, a cure hasn’t been discovered which means the patient has to live
with the disease their entire life. Common examples of chronic diseases are heart conditions, diabetes and cancer. (Side note: Most large pharma companies are shifting their R&D efforts to these kinds of diseases)
help of government subsidies and assistance. (Side note: These drugs are most likely to be outsourced to CDMOs as innovators will not bother setting up manufacturing facilities for them)
are protected by patents which allow the innovator to be the only one marketing the drug for a specified period of time. Generic drugs contain the same chemical ingredients as an innovative drug and can be marketed by other companies after the patent expires.
Insulin, hormones and vaccines are common examples of biologics.
2.3 Biosimilar: A biosimilar is an identical copy of a biologic medication. They are basically the generic versions of biologics.
2.3 Biosimilar: A biosimilar is an identical copy of a biologic medication. They are basically the generic versions of biologics.
2.4 Bioequivalence: In simple terms, two drugs are said to be bioequivalent if they have the same API and have the same intended effect on the body. Bioequivalence studies are conducted to compare if the generic versions of drugs are identical to the
original drug and will affect the body in the same way.
3.1 Intermediate: They are chemical compounds which are manufactured through chemical reactions of basic chemicals. They make up the building blocks for the APIs which are the main ingredients of pharmaceutical drugs.
3.1 Intermediate: They are chemical compounds which are manufactured through chemical reactions of basic chemicals. They make up the building blocks for the APIs which are the main ingredients of pharmaceutical drugs.
3.2 Active Pharmaceutical Ingredient (API): This is the main ingredient in a drug. APIs are manufactured by processing one or more intermediates. APIs are the active ingredient in the drug that produces the intended effect on the human body and are the main
Formulations come in various dosage forms like oral(tablets, capsules, syrups, etc), topical (ointments), injectables,etc.
4.1 Drug Master File (DMF): A DMF is a submission to the US FDA that may be used to provide confidential detailed information about facilities, processes,
4.1 Drug Master File (DMF): A DMF is a submission to the US FDA that may be used to provide confidential detailed information about facilities, processes,
or articles used in the manufacturing, processing, packaging, and storing of one or more human drugs. DMFs are usually filed for APIs. You can download a DMF list from here:
fda.gov
fda.gov
4.2 Veterinary Master File(VMF): A VMF is exactly like DMF except that it is filed for animal drugs and not human drugs. You can download a VMF list from here:
fda.gov
fda.gov
manufacturing standards and drug label. If the FDA approves the NDA, the drug can now be marketed and sold to the public.(Side note: The Animal Pharma equivalent is called NADA - New Animal Drug Application)
Instead, they have to prove that their drug is bioequivalent to the drug that is already on the market. When an ANDA is approved, the drug is added to the Orange book.(Side note: The Animal Pharma equivalent is called ANADA - Abbreviated New Animal Drug Application)
4.5 Orange book: Orange book is a list of drugs and pharmaceuticals that the US FDA has approved as both safe and effective. Although it is commonly called the Orange Book, its formal name is Approved Drug Products with Therapeutic Equivalence Evaluations.
It was first published in 1980 and as of today 41 editions have been released. You can download the latest copy here:
fda.gov
fda.gov
4.6 Purple book: The purple book is like the Orange book except that it contains data about biological drugs rather than chemical drugs. You can download a copy here:
purplebooksearch.fda.gov
purplebooksearch.fda.gov
4.7 Green book: Also exactly like the Orange book, except that it contains data about Animal Drugs. You can download a copy here:
animaldrugsatfda.fda.gov
animaldrugsatfda.fda.gov
Loading suggestions...