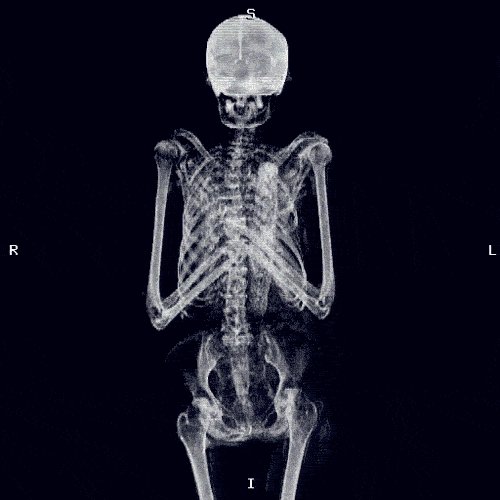
If someone has advanced disease or multiple spinal metastases, there is a risk of increased tumour activity leading to urinary retention, acute compression of the spinal cord or cauda equina.
In such a scenario a GNRH such as Degarelix can be used.
In such a scenario a GNRH such as Degarelix can be used.
Agonists and antagonists of GNRH cause similar side-effects:
Flushing, loss of libido, gynaecomastia, fatigue, low mood and osteoporosis.
These side-effects can have a significant impact on quality of life especially when coupled with the primary symptoms of prostate cancer
Flushing, loss of libido, gynaecomastia, fatigue, low mood and osteoporosis.
These side-effects can have a significant impact on quality of life especially when coupled with the primary symptoms of prostate cancer
Enzalutamide is also used in the castrate resistant setting.
It works as both an androgen receptor antagonists and androgen receptor signalling inhibitor.
It may have less effects on sexual function than other androgen receptor antagonists.
It works as both an androgen receptor antagonists and androgen receptor signalling inhibitor.
It may have less effects on sexual function than other androgen receptor antagonists.
Chemotherapy has been another interesting area of development through the STAMPEDE trials
Historically this was used as a last option. However, in that setting many patients were already very frail and more susceptible to chemo side-effects. Many were not fit for the treatment
Historically this was used as a last option. However, in that setting many patients were already very frail and more susceptible to chemo side-effects. Many were not fit for the treatment
Loading suggestions...