Business overview
- Company has delivered flattish results during the period.
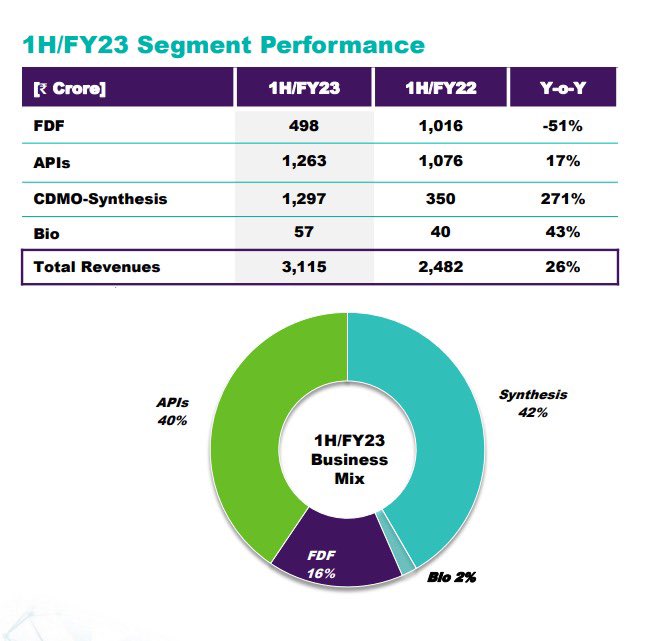
- During the half timeframe, FDF segment have declined by approximately 50% due to weak global agencies but expect a strong recovery from next quarter onwards.
- Company has delivered flattish results during the period.
- During the half timeframe, FDF segment have declined by approximately 50% due to weak global agencies but expect a strong recovery from next quarter onwards.
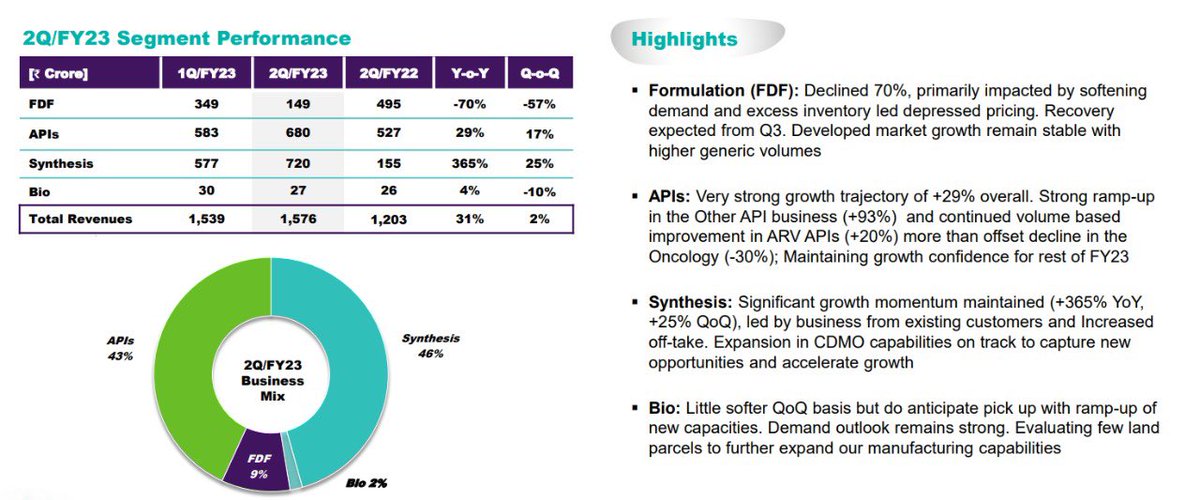
- FDF revenues were mainly dragged by lower ARV business, but strong recovery is expected with higher generic volumes.
- Above 50 active projects at different stages are present in synthesis phase. And they have became suppliers for 4 commercial projects.
- New CEO and COO will take over from Mr. R. Subramani who have stepped down from executive role due to personal reasons.
- New CEO and COO will take over from Mr. R. Subramani who have stepped down from executive role due to personal reasons.
- Under Bios segment, sales jumped by 43% and expect to deliver stronger with company heavy investment in this space.
- Scale,Cost and functionality have been the key drivers for this differential performance.
- Leading to better focus towards product offering and relationship.
- Scale,Cost and functionality have been the key drivers for this differential performance.
- Leading to better focus towards product offering and relationship.
- The net profit stood around 480 crores, due to heavy taxation for the timeline.
- The company has a negative cash flow on operating level due to high inventory levels and supply chain challenges.
- The company has a negative cash flow on operating level due to high inventory levels and supply chain challenges.
- Till now 400 crore Worth of Capex has been incurred.
- Under FDF, unit-2 brownfield capacity was brought online last quarter.
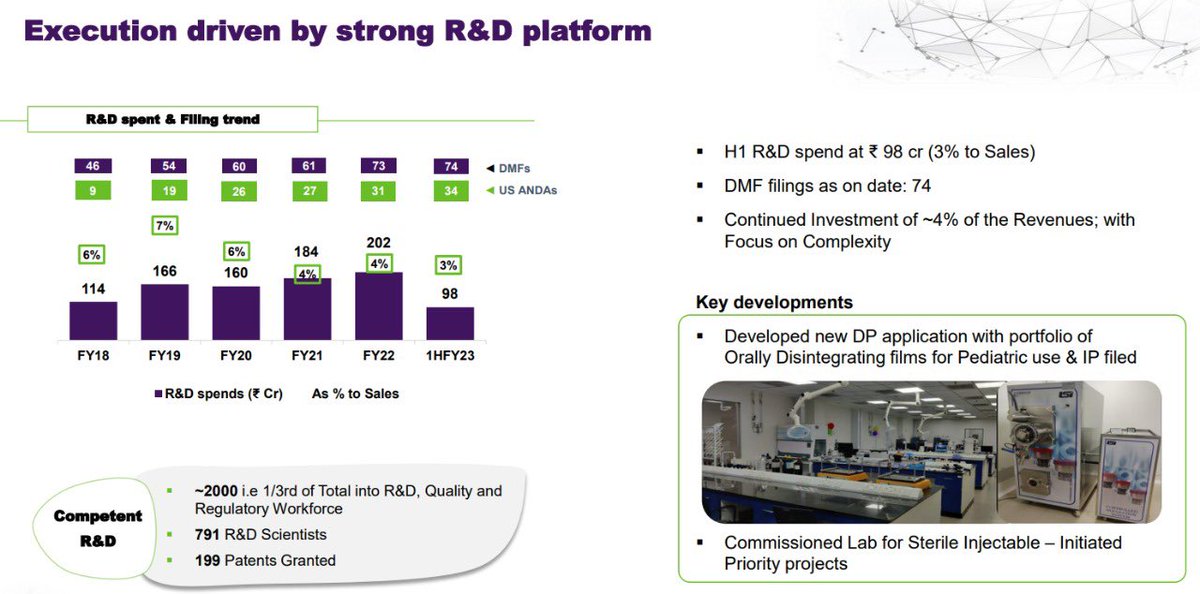
- Under DM filings 3 products were filed in developed markets and they have commissioned R&D lab for sterile injectables.
- Under FDF, unit-2 brownfield capacity was brought online last quarter.
- Under DM filings 3 products were filed in developed markets and they have commissioned R&D lab for sterile injectables.
- In CDMO space, with strong and wider customer base they have initiated plans for new R&D center and 3 manufacturing units.
- All above expected to be completed before 2025.
- All above expected to be completed before 2025.
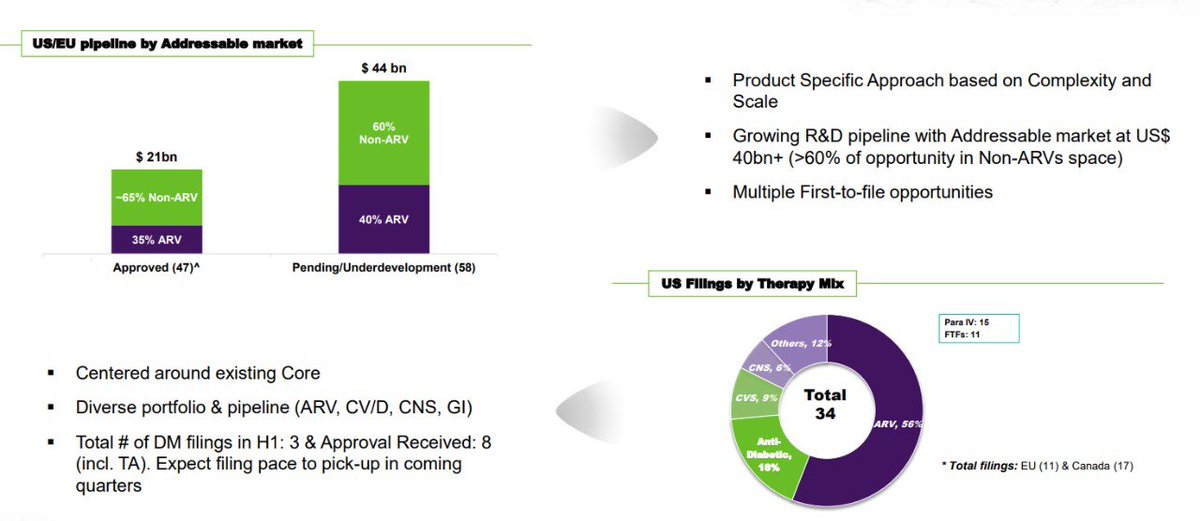
- Till now company has gained 21bn dollars worth of approved pipeline 65% to be non ARV and about 44 billion $ worth is under development in the ratio of 60/40 wherein 60% is for Non ARV.
- They tend to prepare strong pipeline in each segment area to gain market opportunities like.
- Under FDF, they expect to start monetisation in diabetic and CV portfolios.
- Under API, scale up is done in anti-diabetic,CV and PPI.
- Under FDF, they expect to start monetisation in diabetic and CV portfolios.
- Under API, scale up is done in anti-diabetic,CV and PPI.
Loading suggestions...