Infectious Complications post Tx
(Urinary Tract Infections )
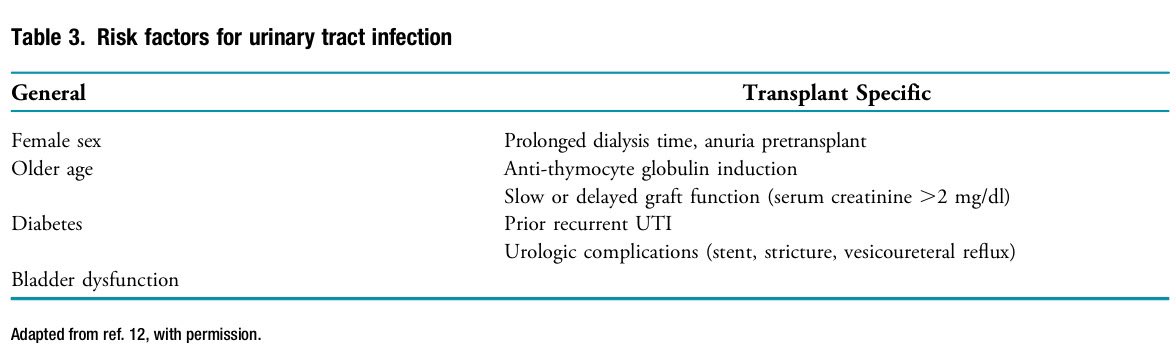
📍UTIs are the most common infection in kidney Tx recipients, causing over 30% of sepsis hospitalizations.
📍Highest incidence: 3-6 months post-Tx.
📍E. coli is the most common pathogen causing UTIs in kidney Tx recipients
📍Asymptomatic bacteriuria: 27% within 1 year, 51% within 3 years post-Tx.
📍For kidney Tx recipients <2 months, management of asymptomatic bacteriuria is not defined as catheters and higher immunosuppression (IS) often masks a true UTI
📍For long-term recipients, treating asymptomatic bacteriuria may lead to resistant organisms without ↓ UTI risk.
📍Antibiotic for UTIs should be based on microbial sensitivity.
📍Treatment: 7-14 days, pyelonephritis: 14-21 days.
📍Early removal of ureteral stents (14-21 days post-Tx)↓infection risks without↑ urologic complications.
📍Lee et al: prophylactic antibiotics at ureteral stent removal may ↓ UTI risk, especially for those not on SMZ/TMP.
📍Prolonged antibiotic prophylaxis can lead to resistant organisms and shown conflicting efficacy in Tx patients.
📍Rosado-Canto et al found single 4-mg dose of fosfomycin, pre-op and before stent removal, significantly↓symptomatic UTIs in first 7 weeks post-Tx.
📍probiotics, cranberry pills, and vaginal estrogen lack evidence for preventing UTIs in kidney Tx
(Urinary Tract Infections )
📍UTIs are the most common infection in kidney Tx recipients, causing over 30% of sepsis hospitalizations.
📍Highest incidence: 3-6 months post-Tx.
📍E. coli is the most common pathogen causing UTIs in kidney Tx recipients
📍Asymptomatic bacteriuria: 27% within 1 year, 51% within 3 years post-Tx.
📍For kidney Tx recipients <2 months, management of asymptomatic bacteriuria is not defined as catheters and higher immunosuppression (IS) often masks a true UTI
📍For long-term recipients, treating asymptomatic bacteriuria may lead to resistant organisms without ↓ UTI risk.
📍Antibiotic for UTIs should be based on microbial sensitivity.
📍Treatment: 7-14 days, pyelonephritis: 14-21 days.
📍Early removal of ureteral stents (14-21 days post-Tx)↓infection risks without↑ urologic complications.
📍Lee et al: prophylactic antibiotics at ureteral stent removal may ↓ UTI risk, especially for those not on SMZ/TMP.
📍Prolonged antibiotic prophylaxis can lead to resistant organisms and shown conflicting efficacy in Tx patients.
📍Rosado-Canto et al found single 4-mg dose of fosfomycin, pre-op and before stent removal, significantly↓symptomatic UTIs in first 7 weeks post-Tx.
📍probiotics, cranberry pills, and vaginal estrogen lack evidence for preventing UTIs in kidney Tx
Infectious Complications post Tx
(Polyomavirus Infection)
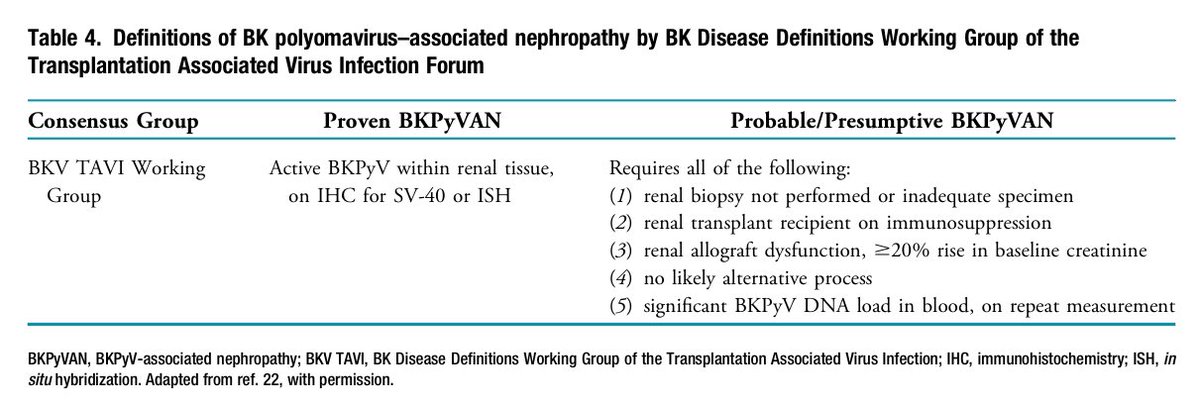
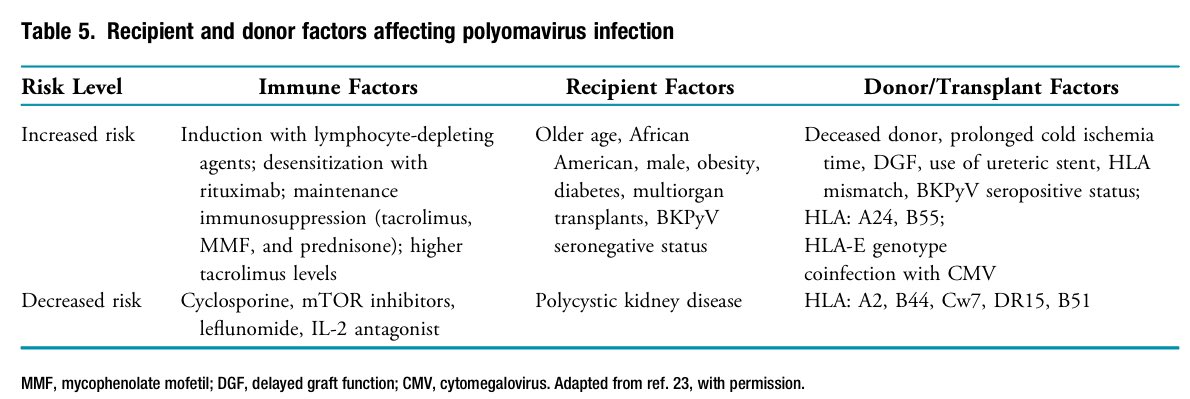
📍In kidney transplant patients, BK polyomavirus reactivation or transmission can cause viruria, viremia, and graft loss.
📍Surveillance and ↓ IS are key in managing BKPyV in kidney Tx recipients.
📍Coinfection with CMV ↑ BKPyV viremia risk.
📍Kidney Tx from HCV-positive donors to HCV-negative recipients are ↑.
📍Molnar et al. found ↑ risk of severe BK viremia in HCV-viremic recipients.
📍No specific antiviral treatment exists for BKPyV.
📍Management relies on early viremia detection and reducing IS, especially MMF
📍Despite reducing IS, 40%-50% of kidney Tx patients may still develop BKPyV nephropathy or acute rejection.
📍IVIG ↑ neutralizing antibodies against BKPyV, and cyclosporine and mTOR inhibitors may help suppress viral replication.
(Polyomavirus Infection)
📍In kidney transplant patients, BK polyomavirus reactivation or transmission can cause viruria, viremia, and graft loss.
📍Surveillance and ↓ IS are key in managing BKPyV in kidney Tx recipients.
📍Coinfection with CMV ↑ BKPyV viremia risk.
📍Kidney Tx from HCV-positive donors to HCV-negative recipients are ↑.
📍Molnar et al. found ↑ risk of severe BK viremia in HCV-viremic recipients.
📍No specific antiviral treatment exists for BKPyV.
📍Management relies on early viremia detection and reducing IS, especially MMF
📍Despite reducing IS, 40%-50% of kidney Tx patients may still develop BKPyV nephropathy or acute rejection.
📍IVIG ↑ neutralizing antibodies against BKPyV, and cyclosporine and mTOR inhibitors may help suppress viral replication.
Infectious Complications post Tx
(Kidney Tx in Patients with HIV Infection)
📍In the era of antiretroviral therapy, HIV patients who receive a kidney Tx have better survival compared to those on dialysis.
📍Zheng et al.'s meta-analysis reported high survival rates (97% at 1 year, 94% at 3 years) for HIV patients post-Tx.
📍Graft survival also good (91% at 1 year, 81% at 3 years).
📍In HIV kidney Tx recipients, 1-year high rejection (33%) & complication (41%)
📍Lymphocyte depletion is safe for HIV kidney Tx recipients with CD4 counts >350 cells/mm³, ↓delayed graft function and↓ death- graft loss rates.
📍Infection rates may be higher in HIV patients receiving T cell-depleting induction.
(Kidney Tx in Patients with HIV Infection)
📍In the era of antiretroviral therapy, HIV patients who receive a kidney Tx have better survival compared to those on dialysis.
📍Zheng et al.'s meta-analysis reported high survival rates (97% at 1 year, 94% at 3 years) for HIV patients post-Tx.
📍Graft survival also good (91% at 1 year, 81% at 3 years).
📍In HIV kidney Tx recipients, 1-year high rejection (33%) & complication (41%)
📍Lymphocyte depletion is safe for HIV kidney Tx recipients with CD4 counts >350 cells/mm³, ↓delayed graft function and↓ death- graft loss rates.
📍Infection rates may be higher in HIV patients receiving T cell-depleting induction.
Malignancy post Tx
📍Post-Tx malignancy is 3-4 times higher than in the general population, highly correlated with the intensity and duration of IS.
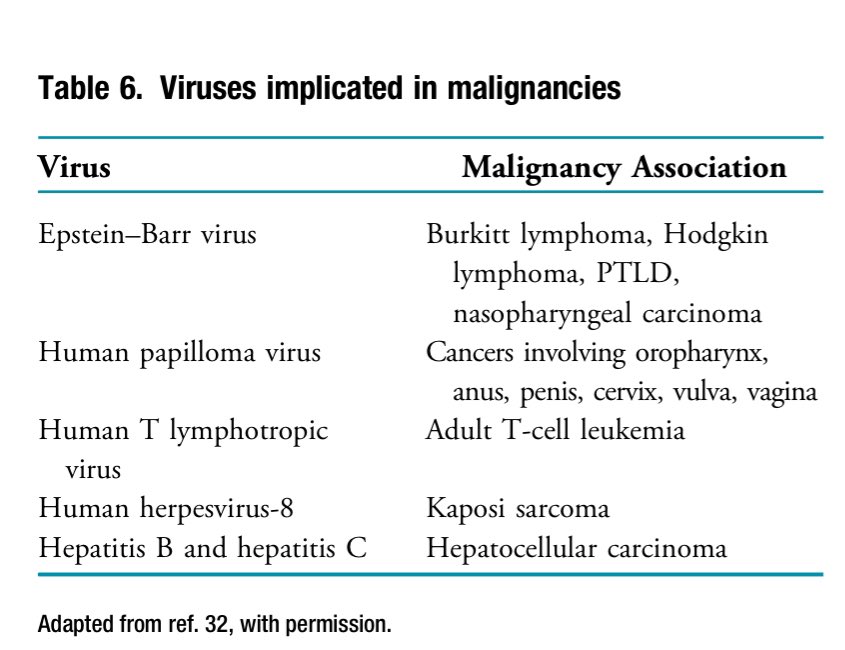
📍Cancer associations with viruses are common in post-Tx patients.
📍Belatacept is linked to PTLD in EBV-seronegative recipients.
📍MMF use in kidney Tx is associated with reduced cancer risk, possibly due to lower acute rejection rates.
📍Switching to mTOR inhibitors:
▪️↓overall cancer risk by 40%
▪️↓nonmelanoma skin cancer by 56%
▪️↓basal cell cancer risk when used de novo
▪️↑ risk of death from CV and infection compared to CNI
Cancer associations with viruses are common, see the listed 👇
📍Post-Tx malignancy is 3-4 times higher than in the general population, highly correlated with the intensity and duration of IS.
📍Cancer associations with viruses are common in post-Tx patients.
📍Belatacept is linked to PTLD in EBV-seronegative recipients.
📍MMF use in kidney Tx is associated with reduced cancer risk, possibly due to lower acute rejection rates.
📍Switching to mTOR inhibitors:
▪️↓overall cancer risk by 40%
▪️↓nonmelanoma skin cancer by 56%
▪️↓basal cell cancer risk when used de novo
▪️↑ risk of death from CV and infection compared to CNI
Cancer associations with viruses are common, see the listed 👇
Malignancy post Tx
(Skin Cancer)
📍AST recommends annual skin surveys for all kidney Tx patients, with more frequent screenings for high-risk patients.
📍Skin cancer occurs in 25% of kidney Tx recipients at 10 years and 60% at 20 years, with SCC 80-fold and basal cell carcinoma 16-fold higher.
📍MMF is associated with a lower risk of SCC compared to azathioprine (OR, 0.45; 95% CI, 0.29 to 0.69).
📍No firm guidelines exist for reducing IS for cutaneous malignancy,
📍Possible approach (SCC): ↓CNI/switch to mTOR inhibitors + consider nicotinamide ( vitamin B₃ )
📍ONTRAC study (nicotinamide 500 mg BID, 1 year): 23% reduction in new skin cancers (fewer BCC, SCC & actinic keratosis (AK)).
📍Phase-2 study: (nicotinamide,6 months) showed non-significant reduction in skin cancers, likely due to short duration and small sample size.
📍Drago et al. study: nicotinamide (kidney & liver Tx) shrunk AK lesions (88%) with complete clearance in 42%, and prevented new AKs compared to placebo (progressed to SCC in some).
📍Nicotinamide should be Use with caution with blood thinners & statins (↑ bleeding & muscle risk).
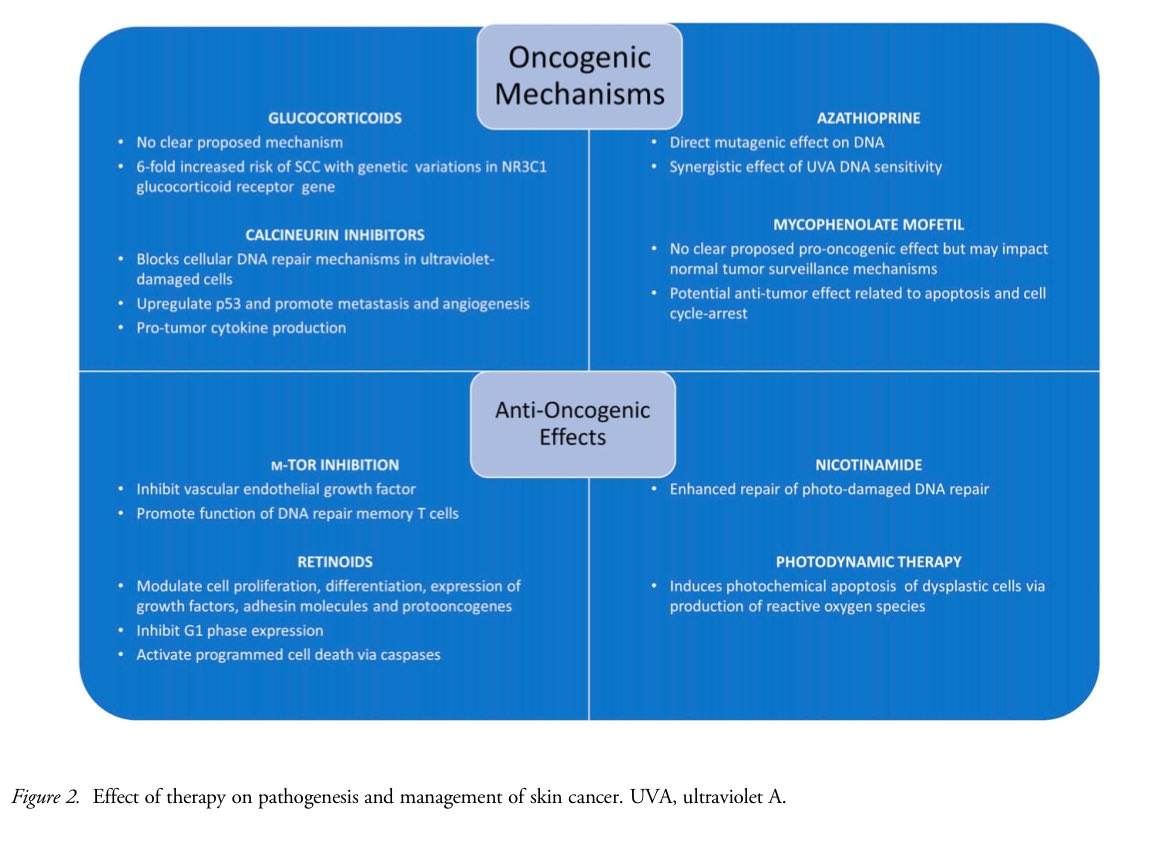
👇 Effect of Therapy on Pathogenesis and Management of Skin Cancer
(Skin Cancer)
📍AST recommends annual skin surveys for all kidney Tx patients, with more frequent screenings for high-risk patients.
📍Skin cancer occurs in 25% of kidney Tx recipients at 10 years and 60% at 20 years, with SCC 80-fold and basal cell carcinoma 16-fold higher.
📍MMF is associated with a lower risk of SCC compared to azathioprine (OR, 0.45; 95% CI, 0.29 to 0.69).
📍No firm guidelines exist for reducing IS for cutaneous malignancy,
📍Possible approach (SCC): ↓CNI/switch to mTOR inhibitors + consider nicotinamide ( vitamin B₃ )
📍ONTRAC study (nicotinamide 500 mg BID, 1 year): 23% reduction in new skin cancers (fewer BCC, SCC & actinic keratosis (AK)).
📍Phase-2 study: (nicotinamide,6 months) showed non-significant reduction in skin cancers, likely due to short duration and small sample size.
📍Drago et al. study: nicotinamide (kidney & liver Tx) shrunk AK lesions (88%) with complete clearance in 42%, and prevented new AKs compared to placebo (progressed to SCC in some).
📍Nicotinamide should be Use with caution with blood thinners & statins (↑ bleeding & muscle risk).
👇 Effect of Therapy on Pathogenesis and Management of Skin Cancer
Malignancy post Tx
(Post-Transplant Lymphoproliferative Disorder)
🔘Risk factors:
📍Early PTLD: EBV-negative young patients with primary EBV infection.
📍Late PTLD: EBV-negative, extranodal involvement, older recipients, expanded-criteria donors, Belatacept use (EBV-negative).
🔘Treatment:
📍Rituximab for CD20-positive PTLD.
📍Chemotherapy for non-responders.
📍Immunosuppression reduction (anti-metabolites stopped, CNI reduced 50%, steroids continued).
📍Newer options for refractory cases (Brentuximab vedotin) but carry↑ risk of rejection.
🔘Prognosis:
📍Lower survival rates than immunocompetent patients (70% at 1 year, 50% at 10 years).
📍Worse prognosis with poor response to rituximab, older age, CNS involvement.
🔘Retransplantation:
📍Option for surviving PTLD Pts with graft loss (minimum 1 year remission).
📍High success rates with proper management (EBV status considered, induction therapy).
📍Increased PTLD recurrence risk compared to de novo PTLD (2.8% vs. 0.8%).
(Post-Transplant Lymphoproliferative Disorder)
🔘Risk factors:
📍Early PTLD: EBV-negative young patients with primary EBV infection.
📍Late PTLD: EBV-negative, extranodal involvement, older recipients, expanded-criteria donors, Belatacept use (EBV-negative).
🔘Treatment:
📍Rituximab for CD20-positive PTLD.
📍Chemotherapy for non-responders.
📍Immunosuppression reduction (anti-metabolites stopped, CNI reduced 50%, steroids continued).
📍Newer options for refractory cases (Brentuximab vedotin) but carry↑ risk of rejection.
🔘Prognosis:
📍Lower survival rates than immunocompetent patients (70% at 1 year, 50% at 10 years).
📍Worse prognosis with poor response to rituximab, older age, CNS involvement.
🔘Retransplantation:
📍Option for surviving PTLD Pts with graft loss (minimum 1 year remission).
📍High success rates with proper management (EBV status considered, induction therapy).
📍Increased PTLD recurrence risk compared to de novo PTLD (2.8% vs. 0.8%).
Bone and Mineral Metabolism
📍Abnormal bone& mineral metabolism common pre and post Tx ,leading to↑ mortality, CVD, bone loss, fractures and ↓ QoL.
📍Chronic IS with steroids significantly influences post-Tx bone disease.
📍Glucocorticoid-sparing regimens ↓ fracture risk by 26% at 1 year and 70% at 3 years post-Tx.
📍Some studies suggest parathyroidectomy (PTx) offers a survival benefit over cinacalcet in dialysis patients, but the best post-Tx approach is unclear.
📍Mathur et al. analyzed data on 5094 kidney Tx recipients treated with cinacalcet (n=4866) or PTx (n=228) for pre-Tx secondary hyperparathyroidism (HPT).
📍Delayed graft function, graft failure, and mortality were similar in both groups.
📍PTx pre-Tx had the lowest risk of tertiary HPT, ↑ in those with dialysis vintage ≥3 years.
📍Persistent HPT is common post-Tx.
📍PreTx cinacalcet dependence and PTH >300 pg/ml predicted persistent HPT.
📍In a 2016 RCT of 30 patients by Cruzado et al. showed: subtotal PTx is superior to cinacalcet for controlling Ca and PTH in post-Tx patients at 1 year.
📍A 5-year follow-up of 24 patients showed normocalcemia in more PTx vs cinacalcet (64% vs. 46%).
📍Cost-utility analysis showed PTx is more cost-effective if cinacalcet is needed >14 months.
📍Abnormal bone& mineral metabolism common pre and post Tx ,leading to↑ mortality, CVD, bone loss, fractures and ↓ QoL.
📍Chronic IS with steroids significantly influences post-Tx bone disease.
📍Glucocorticoid-sparing regimens ↓ fracture risk by 26% at 1 year and 70% at 3 years post-Tx.
📍Some studies suggest parathyroidectomy (PTx) offers a survival benefit over cinacalcet in dialysis patients, but the best post-Tx approach is unclear.
📍Mathur et al. analyzed data on 5094 kidney Tx recipients treated with cinacalcet (n=4866) or PTx (n=228) for pre-Tx secondary hyperparathyroidism (HPT).
📍Delayed graft function, graft failure, and mortality were similar in both groups.
📍PTx pre-Tx had the lowest risk of tertiary HPT, ↑ in those with dialysis vintage ≥3 years.
📍Persistent HPT is common post-Tx.
📍PreTx cinacalcet dependence and PTH >300 pg/ml predicted persistent HPT.
📍In a 2016 RCT of 30 patients by Cruzado et al. showed: subtotal PTx is superior to cinacalcet for controlling Ca and PTH in post-Tx patients at 1 year.
📍A 5-year follow-up of 24 patients showed normocalcemia in more PTx vs cinacalcet (64% vs. 46%).
📍Cost-utility analysis showed PTx is more cost-effective if cinacalcet is needed >14 months.
Metabolic Acidosis and Allograft Function
📍Up to 50% of kidney Tx recipients have metabolic acidosis post-Tx, especially those with eGFR <30
📍Brazier et al. found that ↓ HCO3 levels occur in 40% of kidney Tx recipients and independently predictive of allograft loss.
📍Gojowy et al. reported that 3-year graft survival was 73.7% in kidney Tx recipients with metabolic acidosis compared to 93% in those without.
📍The 3-year survival rate for those with metabolic acidosis was 89.5% compared to 97.4% in those without.
📍Up to 50% of kidney Tx recipients have metabolic acidosis post-Tx, especially those with eGFR <30
📍Brazier et al. found that ↓ HCO3 levels occur in 40% of kidney Tx recipients and independently predictive of allograft loss.
📍Gojowy et al. reported that 3-year graft survival was 73.7% in kidney Tx recipients with metabolic acidosis compared to 93% in those without.
📍The 3-year survival rate for those with metabolic acidosis was 89.5% compared to 97.4% in those without.
Salt and Water Intake & Allograft Outcomes
📍A Cochrane meta-analysis found ↓ sodium intake to <1600 mg/day in CKD patients,↓ systolic/diastolic BP by -6.91/-3.91 mm Hg, but studies were too short to assess long-term outcomes.
📍In kidney Tx, ↓ sodium intake showed modest ↓ in systolic and diastolic BP.
📍(CKD-REIN) study found both low (<0.5 L/day) and high (>2 L/day) water intake were associated with ↑ risk of kidney failure in CKD patients.
📍Neither total water intake nor total urine volume was associated with ↓eGFR or kidney failure in CKD patients.
📍Observational studies do not support ↑ water intake (>3 L/day) in kidney Tx recipients for preserving eGFR at 6 and 12 months.
📍Avoiding dehydration and encouraging adequate fluid intake post kidney Tx is crucial, especially in deceased donor transplants due to early concentrating defects.
📍A Cochrane meta-analysis found ↓ sodium intake to <1600 mg/day in CKD patients,↓ systolic/diastolic BP by -6.91/-3.91 mm Hg, but studies were too short to assess long-term outcomes.
📍In kidney Tx, ↓ sodium intake showed modest ↓ in systolic and diastolic BP.
📍(CKD-REIN) study found both low (<0.5 L/day) and high (>2 L/day) water intake were associated with ↑ risk of kidney failure in CKD patients.
📍Neither total water intake nor total urine volume was associated with ↓eGFR or kidney failure in CKD patients.
📍Observational studies do not support ↑ water intake (>3 L/day) in kidney Tx recipients for preserving eGFR at 6 and 12 months.
📍Avoiding dehydration and encouraging adequate fluid intake post kidney Tx is crucial, especially in deceased donor transplants due to early concentrating defects.
Cardiovascular Health
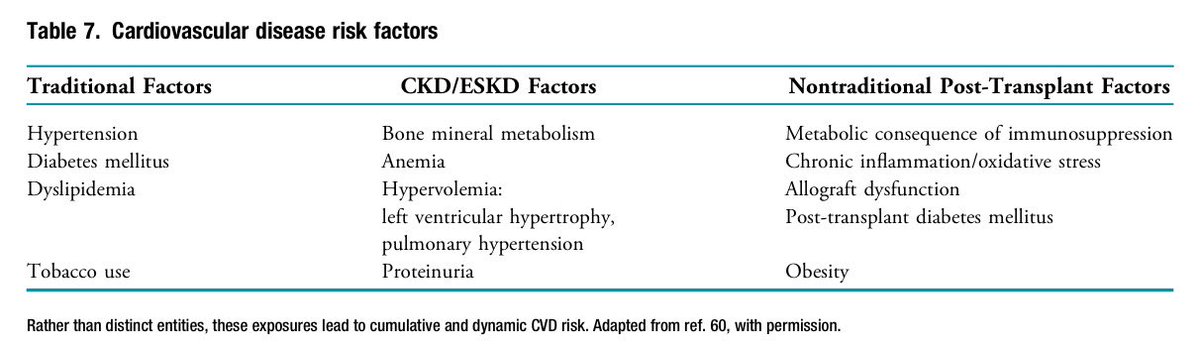
📍Kidney Tx ↓ mortality by ↓ CVD events, but CVD remains the leading cause of death and graft loss in Tx recipients.
📍obstructive sleep apnea are common in CKD and ESKD pts.
📍Every 2% ↓ oxygen saturation ↑ mortality risk by 1.6x.
📍In kidney Tx recipients, worsening sleep disordered correlates with↑BP but doesn't predict long-term allograft dysfunction.
📍The ISCHEMIA-CKD trial found no cardioprotective benefit from an invasive strategy with angiography and revascularization in CKD pts vs medical therapy alone.
📍Post hoc analysis from ISCHEMIA-CKD showed no outcome differences between listed and non-listed kidney Tx candidates assigned to an invasive approach.
📍A national study by Nimmo et al. found no difference in MACE between pre-Tx screening and nonscreening strategies in kidney Tx recipients.
📍Current practice still involves screening asymptomatic Tx candidates for CAD at listing and regular intervals, though its utility is debated.
📍The 🇨🇦-🇦🇺 RCT is testing if eliminating CAD screening in asymptomatic Tx candidates is noninferior to annual testing.
📍A meta-analysis by Siddiqui et al. found no difference in MACE, CV mortality, or all-cause mortality in kidney Tx recipients with CAD who underwent revascularization vs. optimal medical therapy.
📍KDIGO guidelines recommend against routine coronary revascularization to reduce peri-op cardiac events in asymptomatic Tx candidates with known CAD.
📍Kidney Tx ↓ mortality by ↓ CVD events, but CVD remains the leading cause of death and graft loss in Tx recipients.
📍obstructive sleep apnea are common in CKD and ESKD pts.
📍Every 2% ↓ oxygen saturation ↑ mortality risk by 1.6x.
📍In kidney Tx recipients, worsening sleep disordered correlates with↑BP but doesn't predict long-term allograft dysfunction.
📍The ISCHEMIA-CKD trial found no cardioprotective benefit from an invasive strategy with angiography and revascularization in CKD pts vs medical therapy alone.
📍Post hoc analysis from ISCHEMIA-CKD showed no outcome differences between listed and non-listed kidney Tx candidates assigned to an invasive approach.
📍A national study by Nimmo et al. found no difference in MACE between pre-Tx screening and nonscreening strategies in kidney Tx recipients.
📍Current practice still involves screening asymptomatic Tx candidates for CAD at listing and regular intervals, though its utility is debated.
📍The 🇨🇦-🇦🇺 RCT is testing if eliminating CAD screening in asymptomatic Tx candidates is noninferior to annual testing.
📍A meta-analysis by Siddiqui et al. found no difference in MACE, CV mortality, or all-cause mortality in kidney Tx recipients with CAD who underwent revascularization vs. optimal medical therapy.
📍KDIGO guidelines recommend against routine coronary revascularization to reduce peri-op cardiac events in asymptomatic Tx candidates with known CAD.
Cardiovascular Health
(Hypertension and Allograft Outcome)
📍Over 95% of kidney Tx patients remain hypertensive and dependent on antihypertensives.
📍24-hour ABPM is underused.
📍Mallamaci et al. found 25% of kidney Tx recipients had white coat HTN and 12% had masked HTN, with 37% of office BP measurements triggering inappropriate interventions.
📍Korogiannou et al. showed masked HTN in over 30% of kidney Tx recipients, more frequent in males.
📍Wadei et al. reported that disruption in normal diurnal BP patterns leads to nocturnal HTN, a risk factor for poor CV and graft outcomes.
📍Despite findings, universal implementation of ABPM remains a challenge; home BP readings are recommended.
📍The SPRINT trial suggests ↓ BP targets (<120 mm Hg) in CKD pts.
📍The 2021 KDIGO guidelines recommend a target of ≤130/80 mm Hg for kidney Tx recipients.
📍Concerns exist that ↓ systolic BP to <120 mm Hg in kidney Tx may result in AKI and other adverse events.
📍A 2009 meta-analysis by Cross et al. showed that CCBs were associated with a 25% ↓ risk of graft loss and improved eGFR compared to placebo.
📍Studies by Ibrahim et al. found no favorable effect of ARBs on all-cause mortality, graft failure, or serum creatinine doubling in kidney Tx recipients.
📍KDIGO recommends either a dihydropyridine CCB or an ARB as first-line anti HTN in adult kidney Tx recipients.
(Hypertension and Allograft Outcome)
📍Over 95% of kidney Tx patients remain hypertensive and dependent on antihypertensives.
📍24-hour ABPM is underused.
📍Mallamaci et al. found 25% of kidney Tx recipients had white coat HTN and 12% had masked HTN, with 37% of office BP measurements triggering inappropriate interventions.
📍Korogiannou et al. showed masked HTN in over 30% of kidney Tx recipients, more frequent in males.
📍Wadei et al. reported that disruption in normal diurnal BP patterns leads to nocturnal HTN, a risk factor for poor CV and graft outcomes.
📍Despite findings, universal implementation of ABPM remains a challenge; home BP readings are recommended.
📍The SPRINT trial suggests ↓ BP targets (<120 mm Hg) in CKD pts.
📍The 2021 KDIGO guidelines recommend a target of ≤130/80 mm Hg for kidney Tx recipients.
📍Concerns exist that ↓ systolic BP to <120 mm Hg in kidney Tx may result in AKI and other adverse events.
📍A 2009 meta-analysis by Cross et al. showed that CCBs were associated with a 25% ↓ risk of graft loss and improved eGFR compared to placebo.
📍Studies by Ibrahim et al. found no favorable effect of ARBs on all-cause mortality, graft failure, or serum creatinine doubling in kidney Tx recipients.
📍KDIGO recommends either a dihydropyridine CCB or an ARB as first-line anti HTN in adult kidney Tx recipients.
Cardiovascular Health
(Hyperlipidemia)
📍Incidence of dyslipidemia is 50%–80%, prompting lipid-lowering to ↓ CVD.
📍KDIGO recommends all kidney Tx recipients receive cholesterol-lowering agents regardless of LDL level.
📍ALERT Trial, only RCT in Tx recipients, showed a 35% ↓ in cardiac deaths with fluvastatin, but nonsignificant ↓ in primary endpoints.
📍An extension study with 1652 participants reported a 21% ↓ in cardiac events (P = 0.01), but no difference in graft survival.
📍Tailor therapy to the patient, considering statin interactions with CNIs. Avoid combining amlodipine with high-dose simvastatin due to myositis risk.
📍Atorvastatin 80 mg ↓ urinary protein-creatinine ratio by 12.6% in non Tx patients with progressive renal disease.
📍PCSK9 inhibitors used safely with good response in isolated cases.
(Hyperlipidemia)
📍Incidence of dyslipidemia is 50%–80%, prompting lipid-lowering to ↓ CVD.
📍KDIGO recommends all kidney Tx recipients receive cholesterol-lowering agents regardless of LDL level.
📍ALERT Trial, only RCT in Tx recipients, showed a 35% ↓ in cardiac deaths with fluvastatin, but nonsignificant ↓ in primary endpoints.
📍An extension study with 1652 participants reported a 21% ↓ in cardiac events (P = 0.01), but no difference in graft survival.
📍Tailor therapy to the patient, considering statin interactions with CNIs. Avoid combining amlodipine with high-dose simvastatin due to myositis risk.
📍Atorvastatin 80 mg ↓ urinary protein-creatinine ratio by 12.6% in non Tx patients with progressive renal disease.
📍PCSK9 inhibitors used safely with good response in isolated cases.
Cardiovascular Health
(DM, Including Post-Transplant DM (PTDM))
📍Preexisting DM and PTDM are major CVD risk in Tx patients, tripling the risk of fatal and nonfatal CV events compared to non-diabetic recipients.
📍PTDM affects up to 45% of patients with impaired fasting glucose in the first week post-Tx and almost 20% develop PTDM in the first year.
📍IS meds contribute to PTDM; steroids lead to insulin resistance, mTOR inhibitors ↓ insulin sensitivity, and CNIs are toxic to pancreatic beta-cell function.
📍Steroid-avoidance regimens reduce PTDM risk by 30% long-term;
📍conversion from tacrolimus to cyclosporine has shown glycemic improvement in Tx Pts.
📍Older (≥55 years) and obese (BMI ≥30) kidney Tx recipients have a ↑ incidence of PTDM, but the risk is lower with lymphocyte depletion and steroid avoidance.
📍Belatacept has shown ↓ rates of PTDM at 12 months compared to CNIs, but these benefits must be weighed against the potential risk of rejection.
📍Noninsulin antihyperglycemic, such as SGLT2 inhibitors & GLP-1 RA are promising for their cardiorenal protection in non-Tx Pts.
📍The DAPA-CKD trial showed a 39% ↓ in kidney function decline, kidney failure, or death with dapagliflozin. These benefits may extend to kidney Tx recipients.
📍SGLT2 inhibitors may offer benefits in weight loss, uric acid reduction, BP control, and ↓ renal inflammation and fibrosis in kidney Tx recipients, despite infection concerns.
📍Studies show nonsignificant ↓ in BMI, HbA1c, and uric acid with SGLT2 inhibitors in kidney Tx recipients with PTDM.
📍GLP-1 receptor agonists may ↓ weight and improve glycemic control in kidney Tx recipients, further research is needed.
(DM, Including Post-Transplant DM (PTDM))
📍Preexisting DM and PTDM are major CVD risk in Tx patients, tripling the risk of fatal and nonfatal CV events compared to non-diabetic recipients.
📍PTDM affects up to 45% of patients with impaired fasting glucose in the first week post-Tx and almost 20% develop PTDM in the first year.
📍IS meds contribute to PTDM; steroids lead to insulin resistance, mTOR inhibitors ↓ insulin sensitivity, and CNIs are toxic to pancreatic beta-cell function.
📍Steroid-avoidance regimens reduce PTDM risk by 30% long-term;
📍conversion from tacrolimus to cyclosporine has shown glycemic improvement in Tx Pts.
📍Older (≥55 years) and obese (BMI ≥30) kidney Tx recipients have a ↑ incidence of PTDM, but the risk is lower with lymphocyte depletion and steroid avoidance.
📍Belatacept has shown ↓ rates of PTDM at 12 months compared to CNIs, but these benefits must be weighed against the potential risk of rejection.
📍Noninsulin antihyperglycemic, such as SGLT2 inhibitors & GLP-1 RA are promising for their cardiorenal protection in non-Tx Pts.
📍The DAPA-CKD trial showed a 39% ↓ in kidney function decline, kidney failure, or death with dapagliflozin. These benefits may extend to kidney Tx recipients.
📍SGLT2 inhibitors may offer benefits in weight loss, uric acid reduction, BP control, and ↓ renal inflammation and fibrosis in kidney Tx recipients, despite infection concerns.
📍Studies show nonsignificant ↓ in BMI, HbA1c, and uric acid with SGLT2 inhibitors in kidney Tx recipients with PTDM.
📍GLP-1 receptor agonists may ↓ weight and improve glycemic control in kidney Tx recipients, further research is needed.
Cardiovascular Health
(Obesity and Tobacco Use)
Obesity:
📍Common among recipients (60%)
📍Kidney transplant still highly beneficial (66% reduced death risk vs. dialysis)
📍Challenges: ↑ surgical risks, slower recovery, ↑ wound complications
📍Bariatric surgery: Safe for dialysis patients & improves long-term graft survival
Smoking:
📍Major risk factor (30% ↑ graft failure)
📍Linked to cancer, death, worse outcomes
📍As bad for transplants as diabetes
📍Quitting: KDIGO recommends quitting 1 month before waitlist. No smoking cessation drugs studied in transplant recipients yet.
(Obesity and Tobacco Use)
Obesity:
📍Common among recipients (60%)
📍Kidney transplant still highly beneficial (66% reduced death risk vs. dialysis)
📍Challenges: ↑ surgical risks, slower recovery, ↑ wound complications
📍Bariatric surgery: Safe for dialysis patients & improves long-term graft survival
Smoking:
📍Major risk factor (30% ↑ graft failure)
📍Linked to cancer, death, worse outcomes
📍As bad for transplants as diabetes
📍Quitting: KDIGO recommends quitting 1 month before waitlist. No smoking cessation drugs studied in transplant recipients yet.
Cardiovascular Health
(pulmonary HTN )
📍Pre-Tx pulmonary HTN is common, affecting 10% of kidney Tx recipients by 3 years post-Tx.
📍Most cases (97.4%) of pulmonary HTN in kidney Tx recipients are secondary, with ↑ incidence in those with preexisting conditions or longer dialysis duration.
📍Dx of pulmonary HTN in kidney Tx recipients ↑ risk of mortality and graft loss by >2.5 times.
📍Management of pulmonary HTN should focus on identifying reversible causes and optimizing hemodynamics.
📍Ligation of high-flow AVFs may improve pulmonary HTN in kidney Tx recipients
(pulmonary HTN )
📍Pre-Tx pulmonary HTN is common, affecting 10% of kidney Tx recipients by 3 years post-Tx.
📍Most cases (97.4%) of pulmonary HTN in kidney Tx recipients are secondary, with ↑ incidence in those with preexisting conditions or longer dialysis duration.
📍Dx of pulmonary HTN in kidney Tx recipients ↑ risk of mortality and graft loss by >2.5 times.
📍Management of pulmonary HTN should focus on identifying reversible causes and optimizing hemodynamics.
📍Ligation of high-flow AVFs may improve pulmonary HTN in kidney Tx recipients
The Failing Allograft
📍In 2019, 11.9k recipients were listed for retransplantation, making allograft failure the fifth most common cause of ESKD in the 🇺🇸.
📍AST defines a failing allograft as "stable but low function, declining function, and return to RRT."
📍Causes of allograft failure: immunologic and nonimmunologic. Preexisting systemic diseases like CVD, DM, and HTN impact outcomes.
📍Recurrent native kidney disease, especially GN, is the 3rd leading cause of allograft loss.
📍Donor-specific factors like AKI, slow/delayed graft function, donor age, and sex can limit the lifespan of the allograft.
📍Immunologic injury from acute rejection, due to med nonadherence or serious infections, ↑risk of chronic allograft dysfunction.
📍Opportunistic infections, particularly BK nephropathy, can ↑ allograft loss.
📍Managing a failing allograft involves addressing both immunologic and nonimmunologic factors to ↑ graft survival.
📍In 2019, 11.9k recipients were listed for retransplantation, making allograft failure the fifth most common cause of ESKD in the 🇺🇸.
📍AST defines a failing allograft as "stable but low function, declining function, and return to RRT."
📍Causes of allograft failure: immunologic and nonimmunologic. Preexisting systemic diseases like CVD, DM, and HTN impact outcomes.
📍Recurrent native kidney disease, especially GN, is the 3rd leading cause of allograft loss.
📍Donor-specific factors like AKI, slow/delayed graft function, donor age, and sex can limit the lifespan of the allograft.
📍Immunologic injury from acute rejection, due to med nonadherence or serious infections, ↑risk of chronic allograft dysfunction.
📍Opportunistic infections, particularly BK nephropathy, can ↑ allograft loss.
📍Managing a failing allograft involves addressing both immunologic and nonimmunologic factors to ↑ graft survival.
Return to Dialysis and Retransplantation
📍Mortality is ↑ in those who return to dialysis post-Tx.
📍The cumulative incidence of death within 3 years of graft failure in 🇺🇸 is 4.3 per 100 pts.
📍Lack of recognition of graft failure and suboptimal predialysis planning often lead to poor CKD management in failing kidney allograft pts vs. native CKD pts.
📍Pts with failed kidney Tx have worse first-year HD quality metrics in anemia, phosphate, albumin, and vascular access compared native ESKD pts.
📍Over 60% of pts reinitiate dialysis with a central venous catheter.
📍Creating a "failing allograft clinic" could improve management and outcomes.
📍Arshad et al. found recipients in a failing allograft clinic had better discussions on hepatitis B vaccine status, dialysis modality, and retransplant decisions vs. general Tx clinics.
📍Retransplant rates are ↑ among those managed with PD (24%) vs. HD (12%).
📍Rates vary by age and race.
📍Preemptive relisting or Tx is only 15% and has ↓ over time, with ↓ rates among ethnic minorities, men, older individuals, and those with DM.
📍Psychosocial and socioeconomic barriers to ReTx include ↓ education, longer dialysis, longer distance to Tx centers, and residential stressors.
📍Retransplants generally have good outcomes.
📍🇦🇹 Eurotransplant data showed 2nd kidney Tx offers longer survival, especially with short waiting times between Tx.
📍Mortality is ↑ in those who return to dialysis post-Tx.
📍The cumulative incidence of death within 3 years of graft failure in 🇺🇸 is 4.3 per 100 pts.
📍Lack of recognition of graft failure and suboptimal predialysis planning often lead to poor CKD management in failing kidney allograft pts vs. native CKD pts.
📍Pts with failed kidney Tx have worse first-year HD quality metrics in anemia, phosphate, albumin, and vascular access compared native ESKD pts.
📍Over 60% of pts reinitiate dialysis with a central venous catheter.
📍Creating a "failing allograft clinic" could improve management and outcomes.
📍Arshad et al. found recipients in a failing allograft clinic had better discussions on hepatitis B vaccine status, dialysis modality, and retransplant decisions vs. general Tx clinics.
📍Retransplant rates are ↑ among those managed with PD (24%) vs. HD (12%).
📍Rates vary by age and race.
📍Preemptive relisting or Tx is only 15% and has ↓ over time, with ↓ rates among ethnic minorities, men, older individuals, and those with DM.
📍Psychosocial and socioeconomic barriers to ReTx include ↓ education, longer dialysis, longer distance to Tx centers, and residential stressors.
📍Retransplants generally have good outcomes.
📍🇦🇹 Eurotransplant data showed 2nd kidney Tx offers longer survival, especially with short waiting times between Tx.
Immunosuppression Considerations
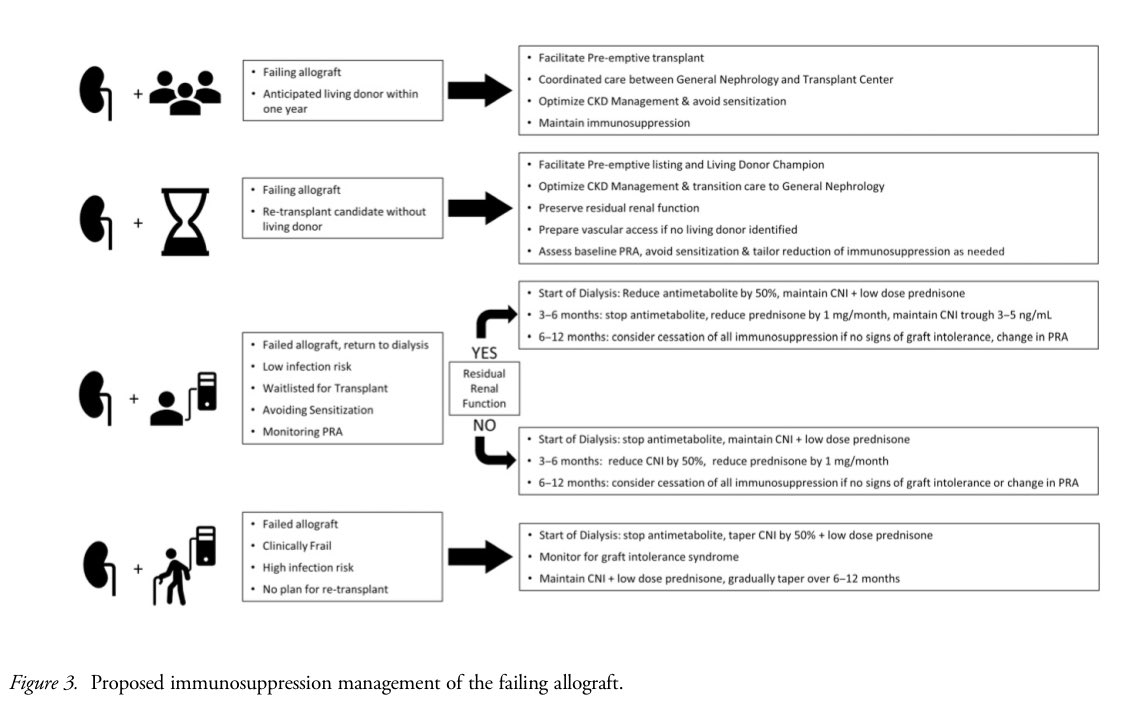
📍Withdrawal of IS is mainly guided by retransplant potential, med-related side effects, infection, frailty, and sensitization.
📍Faster IS withdrawal is favored in pts with ongoing infection, malignancy, or progression of atherosclerotic disease.
📍Lubetzky et al. suggest IS titration should be guided by candidacy for subsequent Tx, residual allograft function, and IS complications.
📍↓ or stopping the antimetabolite first, lowering the CNI trough to preserve residual function, and gradually reducing steroids is recommended for IS tapering.
📍Rapid CNI withdrawal is less favored as maintenance CNI ↓ DSA development, acute symptomatic rejection, and need for allograft nephrectomy.
📍IS weaning ↑ sensitization from 21% to 68% by 6–24 months.
📍Those remaining on IS maintain relatively constant PRA levels.
📍Pts experiencing graft intolerance syndrome may require pulse steroids, oral prednisone taper, and resumption of antimetabolite and CNI.
📍Nephrectomy can lead to sensitization, influenced more by chronic inflammation within the allograft, transfusion needs, and baseline IS rather than the surgery itself.
📍Transitioning back to dialysis after kidney allograft failure is associated with ↑ mortality, emphasizing the need for careful IS management.
👇Proposed Immunosuppression Management of the Failing Allograft
📍Withdrawal of IS is mainly guided by retransplant potential, med-related side effects, infection, frailty, and sensitization.
📍Faster IS withdrawal is favored in pts with ongoing infection, malignancy, or progression of atherosclerotic disease.
📍Lubetzky et al. suggest IS titration should be guided by candidacy for subsequent Tx, residual allograft function, and IS complications.
📍↓ or stopping the antimetabolite first, lowering the CNI trough to preserve residual function, and gradually reducing steroids is recommended for IS tapering.
📍Rapid CNI withdrawal is less favored as maintenance CNI ↓ DSA development, acute symptomatic rejection, and need for allograft nephrectomy.
📍IS weaning ↑ sensitization from 21% to 68% by 6–24 months.
📍Those remaining on IS maintain relatively constant PRA levels.
📍Pts experiencing graft intolerance syndrome may require pulse steroids, oral prednisone taper, and resumption of antimetabolite and CNI.
📍Nephrectomy can lead to sensitization, influenced more by chronic inflammation within the allograft, transfusion needs, and baseline IS rather than the surgery itself.
📍Transitioning back to dialysis after kidney allograft failure is associated with ↑ mortality, emphasizing the need for careful IS management.
👇Proposed Immunosuppression Management of the Failing Allograft
Loading suggestions...