NAFDAC informs healthcare providers and the public of a report of counterfeit Avastin in Nigeria.
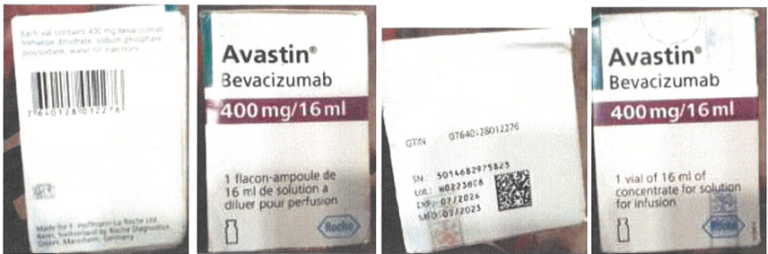
The Marketing Authorization Holder (MAH) Roche reported that an oncologist from a local hospital raised the suspicion of counterfeited material of Avastin Vials 400 mg/16 ml
The Marketing Authorization Holder (MAH) Roche reported that an oncologist from a local hospital raised the suspicion of counterfeited material of Avastin Vials 400 mg/16 ml
The folding box of the unit did not have a NAFDAC number, which is required for sale in Nigeria. The unit was purchased in Lagos at a lower price compared to prices on NCAP (Nigeria Cancer Access Partnership).
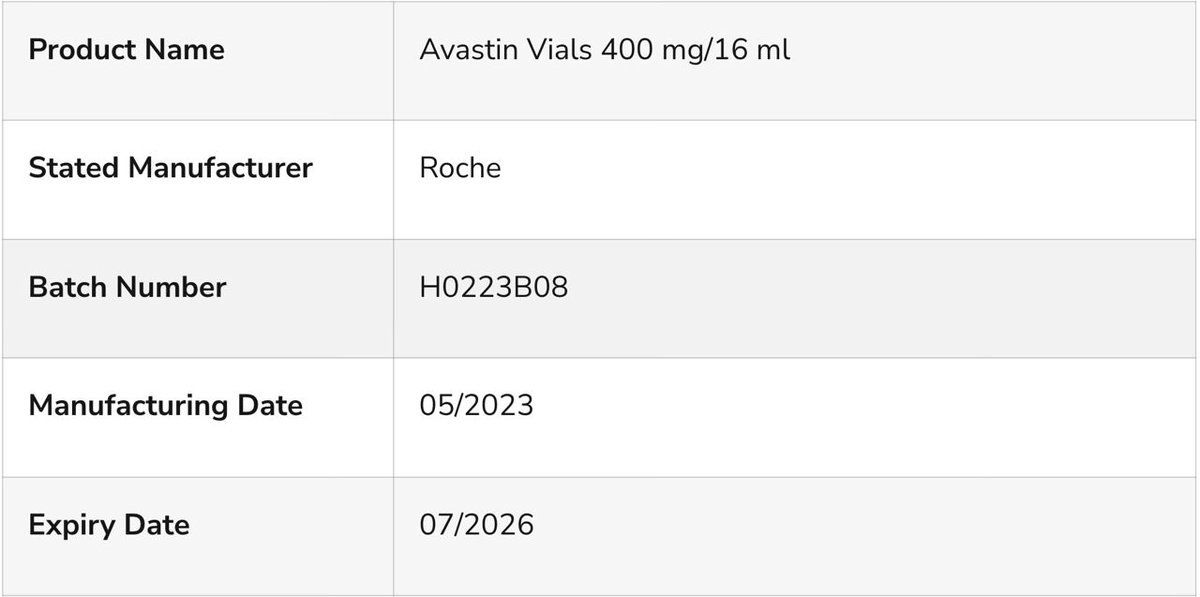
The genuine product of Avastin Vials 400 mg/16 ml, batch H0223B08...
The genuine product of Avastin Vials 400 mg/16 ml, batch H0223B08...
...was distributed by Roche to Vietnam (VN) in July 2020 (expired after 10.02.2022).
The Kaiseraugst/Switzerland Quality Control organization investigated the complaint sample pictures. The displayed complaint sample was compared to retain samples in VN make-up presentation...
The Kaiseraugst/Switzerland Quality Control organization investigated the complaint sample pictures. The displayed complaint sample was compared to retain samples in VN make-up presentation...
...based on the batch no., and to an EFA make-up presentation as the Global Trade Item Number of the folding box belongs to an Avastin 400 mg/16 mL with EFA make-up.
The folding box (including Tamper Evident label) does not correspond to genuine Roche Avastin packaging material.
The folding box (including Tamper Evident label) does not correspond to genuine Roche Avastin packaging material.
An examination of the provided images confirmed clear evidence of counterfeit packaging material in both cases.
AVASTIN is indicated for the treatment of recurrent glioblastoma in adults. It is a tumor-starving (anti-angiogenic) therapy.
AVASTIN is indicated for the treatment of recurrent glioblastoma in adults. It is a tumor-starving (anti-angiogenic) therapy.
Healthcare professionals and consumers are advised to report any suspicion of the sale of substandard and falsified medicines or medical devices to the nearest NAFDAC office, call NAFDAC on 0800-162-3322 or send an email to sf.alert@nafdac.gov.ng
Loading suggestions...